Electrical Stimulation Improves Rat Muscle Dysfunction Caused by Chronic Intermittent Hypoxia-Hypercapnia via Regulation of miRNA-Related Signaling Pathways
- PMID: 27023369
- PMCID: PMC4811440
- DOI: 10.1371/journal.pone.0152525
Electrical Stimulation Improves Rat Muscle Dysfunction Caused by Chronic Intermittent Hypoxia-Hypercapnia via Regulation of miRNA-Related Signaling Pathways
Abstract
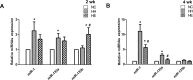
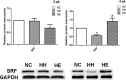
Skeletal muscle dysfunction in chronic obstructive pulmonary disease (COPD) patients is common. Neuromuscular Electrical Stimulation (NMES) is a powerful exercise training that may relieve muscle dysfunction in COPD. This study investigated whether electrical stimulation may have atypical adaptations via activation of miRNA related pathways in counteracting COPD muscle dysfunction. Forty-eight male Sprague-Dawley rats were randomly assigned to 3 groups. With the exception of the rats in the control group, the experimental rats were exposed to chronic intermittent hypoxia-hypercapnia (CIHH) (9∼11%O2,5.5∼6.5%CO2) for 2 or 4 weeks. Electrical stimulation was performed immediately after each CIHH session. Following assessment of the running capacity, biopsy samples were obtained from the gastrocnemius of the rats. The miR-1, miR-133a and miR-133b levels were measured, as well as their related proteins: phosphorylation of Akt (p-AKT), PGC-1alpha (PGC-1α), histone deacetylase 4 (HDAC4) and serum response factor (SRF). Myosin heavy chainIIa (MHCIIa) and myosin heavy chainIIb (MHCIIb) were also measured to assess fiber type changes. After 2 weeks, compared with the controls, only miR-1 and miR-133a were significantly increased (p<0.05) in the exposure group. After 4 weeks, the exposure group exhibited a decreased running distance (p = 0.054) and MHCIIa-to-MHCIIb shift (p<0.05). PGC-1α (p = 0.051), nuclear HDAC4 (p = 0.058), HDAC4, p-AKT, PGC-1α and SRF was also significantly decreased (p<0.05). In contrast, miR-1 and miR-133a were significantly increased (p<0.05). Four weeks of electrical stimulation can partly reversed those changes, and miR-133b exhibited a transient increase after 2 weeks electrical stimulation. Our study indicate miRNAs may have roles in the response of CIHH-impaired muscle to changes during electrical stimulation.
Conflict of interest statement
Figures




Similar articles
-
Electrical stimulation influences chronic intermittent hypoxia-hypercapnia induction of muscle fibre transformation by regulating the microRNA/Sox6 pathway.Sci Rep. 2016 May 20;6:26415. doi: 10.1038/srep26415. Sci Rep. 2016. PMID: 27199002 Free PMC article.
-
Cell Density and Joint microRNA-133a and microRNA-696 Inhibition Enhance Differentiation and Contractile Function of Engineered Human Skeletal Muscle Tissues.Tissue Eng Part A. 2016 Apr;22(7-8):573-83. doi: 10.1089/ten.TEA.2015.0359. Tissue Eng Part A. 2016. PMID: 26891613 Free PMC article.
-
Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation.FASEB J. 2005 May;19(7):786-8. doi: 10.1096/fj.04-2179fje. Epub 2005 Feb 16. FASEB J. 2005. PMID: 15716393
-
Micro-RNAs, Exercise and Cellular Plasticity in Humans: The Impact of Dietary Factors and Hypoxia.Microrna. 2017 Aug 16;6(2):110-124. doi: 10.2174/2211536606666170519133144. Microrna. 2017. PMID: 28523996 Review.
-
MiR-210--micromanager of the hypoxia pathway.Trends Mol Med. 2010 May;16(5):230-7. doi: 10.1016/j.molmed.2010.03.004. Epub 2010 Apr 29. Trends Mol Med. 2010. PMID: 20434954 Free PMC article. Review.
Cited by
-
Neuro-Muscular Dentistry: the "diamond" concept of electro-stimulation potential for stomato-gnathic and oro-dental conditions.Head Face Med. 2021 Jan 26;17(1):2. doi: 10.1186/s13005-021-00257-3. Head Face Med. 2021. PMID: 33499906 Free PMC article. Review.
-
Mitochondrial quality control: a pathophysiological mechanism and potential therapeutic target for chronic obstructive pulmonary disease.Front Pharmacol. 2025 Jan 3;15:1474310. doi: 10.3389/fphar.2024.1474310. eCollection 2024. Front Pharmacol. 2025. PMID: 39830343 Free PMC article. Review.
-
Melatonin Protects Rabbit Somatic Cell Nuclear Transfer (SCNT) Embryos from Electrofusion Damage.Sci Rep. 2020 Feb 10;10(1):2186. doi: 10.1038/s41598-020-59161-6. Sci Rep. 2020. PMID: 32042116 Free PMC article.
-
Hypercapnia Regulates Gene Expression and Tissue Function.Front Physiol. 2020 Nov 20;11:598122. doi: 10.3389/fphys.2020.598122. eCollection 2020. Front Physiol. 2020. PMID: 33329047 Free PMC article. Review.
-
Impact of dcEF on microRNA profiles in glioblastoma and exosomes using a novel microfluidic bioreactor.Biomicrofluidics. 2024 Dec 27;18(6):064106. doi: 10.1063/5.0228901. eCollection 2024 Dec. Biomicrofluidics. 2024. PMID: 39742343 Free PMC article.
References
-
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. American journal of respiratory and critical care medicine. 1996. March;153(3):976–80. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

