Stabilization of Microtubule-Unbound Tau via Tau Phosphorylation at Ser262/356 by Par-1/MARK Contributes to Augmentation of AD-Related Phosphorylation and Aβ42-Induced Tau Toxicity
- PMID: 27023670
- PMCID: PMC4811436
- DOI: 10.1371/journal.pgen.1005917
Stabilization of Microtubule-Unbound Tau via Tau Phosphorylation at Ser262/356 by Par-1/MARK Contributes to Augmentation of AD-Related Phosphorylation and Aβ42-Induced Tau Toxicity
Abstract
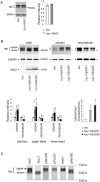
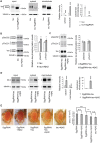
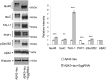
Abnormal accumulation of the microtubule-interacting protein tau is associated with neurodegenerative diseases including Alzheimer's disease (AD). β-amyloid (Aβ) lies upstream of abnormal tau behavior, including detachment from microtubules, phosphorylation at several disease-specific sites, and self-aggregation into toxic tau species in AD brains. To prevent the cascade of events leading to neurodegeneration in AD, it is essential to elucidate the mechanisms underlying the initial events of tau mismetabolism. Currently, however, these mechanisms remain unclear. In this study, using transgenic Drosophila co-expressing human tau and Aβ, we found that tau phosphorylation at AD-related Ser262/356 stabilized microtubule-unbound tau in the early phase of tau mismetabolism, leading to neurodegeneration. Aβ increased the level of tau detached from microtubules, independent of the phosphorylation status at GSK3-targeted SP/TP sites. Such mislocalized tau proteins, especially the less phosphorylated species, were stabilized by phosphorylation at Ser262/356 via PAR-1/MARK. Levels of Ser262 phosphorylation were increased by Aβ42, and blocking this stabilization of tau suppressed Aβ42-mediated augmentation of tau toxicity and an increase in the levels of tau phosphorylation at the SP/TP site Thr231, suggesting that this process may be involved in AD pathogenesis. In contrast to PAR-1/MARK, blocking tau phosphorylation at SP/TP sites by knockdown of Sgg/GSK3 did not reduce tau levels, suppress tau mislocalization to the cytosol, or diminish Aβ-mediated augmentation of tau toxicity. These results suggest that stabilization of microtubule-unbound tau by phosphorylation at Ser262/356 via the PAR-1/MARK may act in the initial steps of tau mismetabolism in AD pathogenesis, and that such tau species may represent a potential therapeutic target for AD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–6. - PubMed
-
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293(5534):1487–91. - PubMed
-
- Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293(5534):1491–5. - PubMed
-
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43(3):321–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

