Patterns and Economic Burden of Hospitalizations and Exacerbations Among Patients Diagnosed with Idiopathic Pulmonary Fibrosis
- PMID: 27023695
- PMCID: PMC10398274
- DOI: 10.18553/jmcp.2016.22.4.414
Patterns and Economic Burden of Hospitalizations and Exacerbations Among Patients Diagnosed with Idiopathic Pulmonary Fibrosis
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is a rare and fatal restrictive respiratory disease under the idiopathic lung disease (ILD) class. IPF is a form of chronic, progressive fibrosing interstitial pneumonia and has more scarring, less inflammation, and poorer prognosis than most other ILD forms. Exacerbation of IPF is rapid, with unpredictable deterioration of lung function, and is associated with short-term mortality. The American Thoracic Society (ATS) evidence-based guidelines for diagnosis and management of IPF reports that the incidence of acute exacerbations is between 5%-10%. Limited real-world evidence has been identified in the United States that assesses patterns of hospitalization, exacerbation of IPF, and the associated economic burden.
Objectives: To (a) characterize patients newly diagnosed with IPF and (b) examine incidence rates and costs of all-cause hospitalizations, IPF-related hospitalizations, and exacerbations.
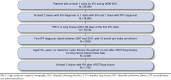
Methods: A retrospective analysis was performed with a national commercial claims database from calendar years 2006 to 2011. Newly diagnosed IPF patients were identified with either ≥ 2 claims for idiopathic fibrosing alveolitis (IFA) or ≥ 1 claim for IFA and ≥ 1 claim for postinflammatory pulmonary fibrosis and a lung biopsy or thoracic high-resolution computed tomography within 90 days of the first claim for IFA (index date). IPF-related hospitalizations and possible IPF exacerbations were defined based on diagnoses recorded on event claims. Frequency, incidence rate, duration of events, and associated costs from the third-party payer's perspective were estimated.
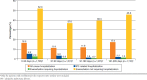
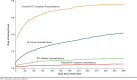
Results: Among 1,735 identified IPF patients, 38.6% had at least 1 all-cause hospitalization; 10.8% had IPF-related hospitalizations; 4.6% had suspected IPF exacerbations leading to hospitalization; and 72.1% had suspected IPF exacerbations leading to urgent outpatient visits during the 1-year post-index period. Incident rates for these 4 events were 83 (95% CI = 79-88), 17 (95% CI = 14-19), 7 (95% CI = 6-9), and 277 (95% CI = 269-286) per 100 person-years, respectively. Average costs per event were $13,987 (SD = $41,988), $16,812 (SD = $66,399), $14,731 (SD = $85,468), and $444 (SD = $1,481), respectively.
Conclusions: Hospitalizations and possible exacerbations among patients with IPF were costly. Appropriate management of IPF needs to be considered to help slow IPF disease progression.
Disclosures: Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) provided funding for this study. Yu and Devercelli are currently salaried employees of BIPI. Wu, Chuang, Wang, Pan, and Benjamin are currently employees of Evidera, which provides consulting and other research services to pharmaceutical, device, government, and nongovernment organizations. In their salaried positions, they work with a variety of companies and organizations and are precluded from receiving payment or honoraria directly from these organizations for services rendered. Evidera received funding from BIPI to conduct the analysis. Coultas was previously a paid consultant of BIPI. The contents do not represent the views of the Department of Veterans Affairs or the U.S. government. This manuscript does not contain clinical studies or patient data. The authors have full control of all primary data, and they agree to allow the journal to review their data if requested. All authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors, and they are fully responsible for all content and editorial decisions and were involved at all stages of manuscript development. The manuscript was drafted by Benjamin, Wu, and Yu and revised by Wang, Pan, Yu, Coultas, and Devercelli. The study was designed by Yu, Wu, Chuang, Wang, Benjamin, and Coultas. Statistical analysis was conducted by Wu, Chuang, and Wang. Senior review was provided by Coultas and Devercelli.
Conflict of interest statement
Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) provided funding for this study. Yu and Devercelli are currently salaried employees of BIPI. Wu, Chuang, Wang, Pan, and Benjamin are currently employees of Evidera, which provides consulting and other research services to pharmaceutical, device, government, and nongovernment organizations. In their salaried positions, they work with a variety of companies and organizations and are precluded from receiving payment or honoraria directly from these organizations for services rendered. Evidera received funding from BIPI to conduct the analysis. Coultas was previously a paid consultant of BIPI. The contents do not represent the views of the Department of Veterans Affairs or the U.S. government.
This manuscript does not contain clinical studies or patient data. The authors have full control of all primary data, and they agree to allow the journal to review their data if requested.
All authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors, and they are fully responsible for all content and editorial decisions and were involved at all stages of manuscript development.
The manuscript was drafted by Benjamin, Wu, and Yu and revised by Wang, Pan, Yu, Coultas, and Devercelli. The study was designed by Yu, Wu, Chuang, Wang, Benjamin, and Coultas. Statistical analysis was conducted by Wu, Chuang, and Wang. Senior review was provided by Coultas and Devercelli.
Figures
References
-
- Agarwal R, Jindal SK. Acute exacerbation of idiopathic pulmonary fibrosis: a systematic review. Eur J Intern Med. 2008;19(4):227-35. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources