Identification of a Fragment-Based Scaffold that Inhibits the Glycosyltransferase WaaG from Escherichia coli
- PMID: 27025525
- PMCID: PMC4810412
- DOI: 10.3390/antibiotics5010010
Identification of a Fragment-Based Scaffold that Inhibits the Glycosyltransferase WaaG from Escherichia coli
Abstract
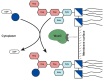
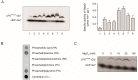
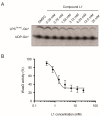
WaaG is a glycosyltransferase that is involved in the biosynthesis of lipopolysaccharide in Gram-negative bacteria. Inhibitors of WaaG are highly sought after as they could be used to inhibit the biosynthesis of the core region of lipopolysaccharide, which would improve the uptake of antibiotics. Herein, we establish an activity assay for WaaG using (14)C-labeled UDP-glucose and LPS purified from a ∆waaG strain of Escherichia coli. We noted that addition of the lipids phosphatidylglycerol (PG) and cardiolipin (CL), as well as the detergent 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) increased activity. We then use the assay to determine if three molecular scaffolds, which bind to WaaG, could inhibit its activity in vitro. We show that 4-(2-amino-1,3-thiazol-4-yl)phenol inhibits WaaG (IC50 1.0 mM), but that the other scaffolds do not. This study represents an important step towards an inhibitor of WaaG by fragment-based lead discovery.
Keywords: Gram-negative bacteria; fragment-based lead discovery; glucosyltransferase; lipopolysaccharide; scaffold.
Figures


References
-
- Liu A., Tran L., Becket E., Lee K., Chinn L., Park E., Tran K., Miller J.H. Antibiotic sensitivity profiles determined with an Escherichia coli gene knockout collection: Generating an antibiotic bar code. Antimicrob. Agents Chemother. 2010;54:1393–1403. doi: 10.1128/AAC.00906-09. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

