Comparison of 2-Ethyl-Cyanoacrylate and 2-Butyl-Cyanoacrylate for Use on the Calvaria of CD1 Mice
- PMID: 27025812
- PMCID: PMC4783639
Comparison of 2-Ethyl-Cyanoacrylate and 2-Butyl-Cyanoacrylate for Use on the Calvaria of CD1 Mice
Abstract
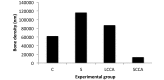
Short-chain cyanoacrylates (SCCA), such as ethyl-2-cyanoacrylate (KrazyGlue, Aron Alpha, Columbus, OH) are commonly used as commercial fast-acting glues. Although once used in clinical medicine as skin adhesives, these products caused tissue toxicity and thus their use in live tissue was discontinued. SCCA were replaced by longer-chain versions (LCCA), such as butyl-cyanoacrylate (Vetbond, 3M, St Paul, Minnesota), which were found to be less toxic than the short-chain formulations. Some researchers prefer to use SCCA due to the belief that they create a stronger bond than do the longer-chain counterparts. In survival surgeries, we compared the bone thickness, bone necrosis, fibrosis, inflammation, and bone regeneration in the calvaria of control (naïve), surgery-only, SCCA-treated, and LCCA-treated mice (n = 20 per group). At 1 and 14 d after surgery, all mice except those treated with SCCA showed statistically similar bone measurements to those of the naive control group. The SCCA group had significantly less bone regeneration than did all other groups. These results suggest that the application of SCCA causes bone damage resulting in the loss of bone regeneration. This finding may assist investigators in choosing a tissue glue for their studies and may support the IACUC in advocating the use of pharmaceutical-grade tissue glues.
Figures


Similar articles
-
Histotoxicity of cyanoacrylate tissue adhesives. A comparative study.Arch Otolaryngol Head Neck Surg. 1990 May;116(5):546-50. doi: 10.1001/archotol.1990.01870050046004. Arch Otolaryngol Head Neck Surg. 1990. PMID: 2183824
-
Comparison of the bacteriostatic effects, corneal cytotoxicity, and the ability to seal corneal incisions among three different tissue adhesives.Cornea. 2007 Dec;26(10):1228-34. doi: 10.1097/ICO.0b013e3181506129. Cornea. 2007. PMID: 18043181
-
Cyanoacrylate in nerve repair: transient cytotoxic effect.Int J Oral Maxillofac Surg. 2010 Jul;39(7):705-12. doi: 10.1016/j.ijom.2010.03.008. Int J Oral Maxillofac Surg. 2010. PMID: 20434310
-
Cyanoacrylates for skin closure.Dermatol Clin. 2005 Apr;23(2):193-8. doi: 10.1016/j.det.2004.09.003. Dermatol Clin. 2005. PMID: 15837150 Review.
-
Surgical applications of cyanoacrylate adhesives: a review of toxicity.ANZ J Surg. 2007 Apr;77(4):209-13. doi: 10.1111/j.1445-2197.2007.04020.x. ANZ J Surg. 2007. PMID: 17388821 Review.
Cited by
-
Tissue Adhesives: From Research to Clinical Translation.Nano Today. 2021 Feb;36:101049. doi: 10.1016/j.nantod.2020.101049. Epub 2020 Dec 20. Nano Today. 2021. PMID: 33425002 Free PMC article.
-
Comparison of peripheral nerve repair using ethyl-cyanoacrylate and conventional suture technique in a rat sciatic nerve injury model.Acta Orthop Traumatol Turc. 2020 May;54(3):330-336. doi: 10.5152/j.aott.2020.03.156. Acta Orthop Traumatol Turc. 2020. PMID: 32544069 Free PMC article.
-
Application and outlook of topical hemostatic materials: a narrative review.Ann Transl Med. 2021 Apr;9(7):577. doi: 10.21037/atm-20-7160. Ann Transl Med. 2021. PMID: 33987275 Free PMC article. Review.
-
Endoscopic treatment of bronchopleural fistula using ethyl-2-cyanoacrylate: A report of two cases.Respir Med Case Rep. 2020 Jun 10;30:101123. doi: 10.1016/j.rmcr.2020.101123. eCollection 2020. Respir Med Case Rep. 2020. PMID: 32577364 Free PMC article.
-
Comparison between N-butyl cyanoacrylate tissue adhesive and Ethilon nylon sutures in extraoral maxillofacial incisions: A randomized prospective study.J Oral Biol Craniofac Res. 2019 Jul-Sep;9(3):173-178. doi: 10.1016/j.jobcr.2019.04.002. Epub 2019 Apr 16. J Oral Biol Craniofac Res. 2019. PMID: 31049280 Free PMC article.
References
-
- Agterberg MJ, Spoelstra EN, van der Wijst S, Brakkee JH, Wiegant VM, Hamelink R, Brouns K, Westerink BH, Remie R. 2010. Evaluation of temperature rise and bonding strength in cements used for permanent head attachments in rats and mice. Lab Anim 44:264–270. - PubMed
-
- Animal Welfare Act as Amended. 2008. 7 USC §2131–2159.
-
- Chou SN. 1977. Use of cyanoacrylate. J Neurosurg 46:266. - PubMed
-
- Clarke TF. 2011. Cyanoacrylate glue burn in a child—lessons to be learned. J Plast Reconstr Aesthet Surg 64:e170–e173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
