12 Weeks of Daclatasvir in Combination With Sofosbuvir for HIV-HCV Coinfection (ALLY-2 Study): Efficacy and Safety by HIV Combination Antiretroviral Regimens
- PMID: 27025835
- PMCID: PMC4885650
- DOI: 10.1093/cid/ciw163
12 Weeks of Daclatasvir in Combination With Sofosbuvir for HIV-HCV Coinfection (ALLY-2 Study): Efficacy and Safety by HIV Combination Antiretroviral Regimens
Abstract
Background: Highly effective hepatitis C virus (HCV) direct-acting antiviral therapies that do not require modification of human immunodeficiency virus (HIV) antiretroviral regimens are needed. We evaluated the efficacy and safety of daclatasvir + sofosbuvir (DCV + SOF) for 12 weeks by antiretroviral (ARV) regimen in HIV-HCV-coinfected patients.
Methods: In the randomized, open-label ALLY-2 study, HIV-HCV-coinfected patients received 8 or 12 weeks of once-daily DCV 60 mg (dose-adjusted as-necessary for concomitant ARVs) + SOF 400 mg. Results were stratified by ARV class for the 151 patients who received 12 weeks of DCV + SOF.
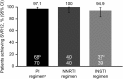
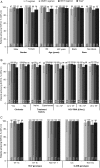
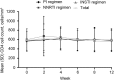
Results: Fifty-one patients were HCV treatment experienced, 100 were treatment naive, 89% male and 33% black. HCV genotypes were: genotype 1a (GT1a; 69%), GT1b (15%), GT2 (8%), GT3 (6%), and GT4 (2%). Sustained virologic response 12 weeks post-treatment (SVR12) was 97% and was similar across ARV regimens (P = .774): protease inhibitor-based, 97% (95% confidence interval [CI], 90%-99.7%); nonnucleoside reverse transcriptase inhibitor-based, 100% (95% CI, 91%-100%); and integrase inhibitor based, 95% (95% CI, 83%-99.4%). SVR12 among patients receiving either tenofovir disoproxil fumarate or abacavir as part of their antiretroviral therapy regimen was 98% (95% CI, 93%-99.5%) and 100% (95% CI, 85%-100%), respectively. Age, gender, race, cirrhosis, HCV treatment history, GT , and baseline HCV RNA did not affect SVR12. No discontinuations were attributed to treatment-related adverse events.
Conclusions: DCV + SOF x12 weeks is a highly efficacious, all-oral, pan-GT HCV treatment for HIV-HCV coinfected patients across a broad range of ARV regimens.
Clinical trials registration: NCT02032888.
Keywords: HIV-HCV; antiretroviral therapy; coinfection; daclatasvir; sofosbuvir.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Similar articles
-
Daclatasvir and Sofosbuvir Versus Sofosbuvir and Ribavirin in Patients with Chronic Hepatitis C Coinfected with HIV: A Matching-adjusted Indirect Comparison.Clin Ther. 2016 Feb;38(2):404-12. doi: 10.1016/j.clinthera.2015.12.017. Epub 2016 Feb 3. Clin Ther. 2016. PMID: 26839044
-
Effectiveness of All-Oral Antiviral Regimens in 996 Human Immunodeficiency Virus/Hepatitis C Virus Genotype 1-Coinfected Patients Treated in Routine Practice.Clin Infect Dis. 2017 Jun 15;64(12):1711-1720. doi: 10.1093/cid/cix111. Clin Infect Dis. 2017. PMID: 28199525
-
Sustained virologic response and changes in liver fibrosis parameters following 12-wk administration of generic sofosbuvir and daclatasvir in HIV/HCV-coinfected patients with HCV genotype 4 infection.Trans R Soc Trop Med Hyg. 2020 Apr 8;114(4):232-240. doi: 10.1093/trstmh/trz120. Trans R Soc Trop Med Hyg. 2020. PMID: 31925434
-
Daclatasvir plus sofosbuvir, with or without ribavirin, in real-world patients with HIV-HCV coinfection and advanced liver disease.Antivir Ther. 2017;22(3):225-236. doi: 10.3851/IMP3108. Epub 2016 Nov 15. Antivir Ther. 2017. PMID: 27845298
-
Efficacy and safety of direct acting antiviral regimens for hepatitis C virus and human immunodeficiency virus co-infection: systematic review and network meta-analysis.J Gastroenterol Hepatol. 2020 Sep;35(9):1477-1487. doi: 10.1111/jgh.15051. Epub 2020 Apr 15. J Gastroenterol Hepatol. 2020. PMID: 32246857
Cited by
-
Development of a Robust UPLC Method for Simultaneous Determination of a Novel Combination of Sofosbuvir and Daclatasvir in Human Plasma: Clinical Application to Therapeutic Drug Monitoring.Int J Anal Chem. 2018 Oct 21;2018:6535816. doi: 10.1155/2018/6535816. eCollection 2018. Int J Anal Chem. 2018. PMID: 30420886 Free PMC article.
-
Contemporary HCV pangenotypic DAA treatment protocols are exclusionary to real world HIV-HCV co-infected patients.BMC Infect Dis. 2019 May 3;19(1):378. doi: 10.1186/s12879-019-3974-7. BMC Infect Dis. 2019. PMID: 31053098 Free PMC article.
-
Daclatasvir: A Review in Chronic Hepatitis C.Drugs. 2016 Sep;76(14):1381-91. doi: 10.1007/s40265-016-0632-x. Drugs. 2016. PMID: 27550544 Review.
-
Efficacy of Sovodak in the Management of Patients Co-infected with HIV/HCV.Adv Pharm Bull. 2020 Sep;10(4):662-665. doi: 10.34172/apb.2020.080. Epub 2020 Aug 9. Adv Pharm Bull. 2020. PMID: 33072543 Free PMC article.
-
Effectiveness of direct-acting antivirals for hepatitis C virus infection in hepatitis C/HIV coinfected individuals: A multicenter study.Medicine (Baltimore). 2020 Jul 24;99(30):e21270. doi: 10.1097/MD.0000000000021270. Medicine (Baltimore). 2020. PMID: 32791706 Free PMC article.
References
-
- Bica I, McGovern B, Dhar R et al. . Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis 2001; 32:492–7. - PubMed
-
- Centers for Disease Control and Prevention. HIV and viral hepatitis factsheet. 2014. Available at: www.cdc.gov/hiv/pdf/library_factsheets_HIV_and_viral_Hepatitis.pdf Accessed August 2015.
-
- Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf Updated April 2015. Accessed November 2015.
-
- European AIDS Clinical Society. Guidelines Version 8.0. October 2015. Available at: http://www.eacsociety.org/files/guidelines-8.0-english.pdf Accessed November 2015.
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

