Neuronal Wiskott-Aldrich syndrome protein regulates TGF-β1-mediated lung vascular permeability
- PMID: 27025963
- PMCID: PMC4904290
- DOI: 10.1096/fj.201600102R
Neuronal Wiskott-Aldrich syndrome protein regulates TGF-β1-mediated lung vascular permeability
Abstract
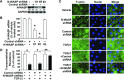
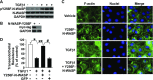
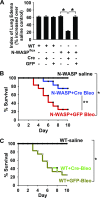
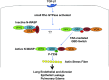
TGF-β1 induces an increase in paracellular permeability and actin stress fiber formation in lung microvascular endothelial and alveolar epithelial cells via small Rho GTPase. The molecular mechanism involved is not fully understood. Neuronal Wiskott-Aldrich syndrome protein (N-WASP) has an essential role in actin structure dynamics. We hypothesized that N-WASP plays a critical role in these TGF-β1-induced responses. In these cell monolayers, we demonstrated that N-WASP down-regulation by short hairpin RNA prevented TGF-β1-mediated disruption of the cortical actin structure, actin stress filament formation, and increased permeability. Furthermore, N-WASP down-regulation blocked TGF-β1 activation mediated by IL-1β in alveolar epithelial cells, which requires actin stress fiber formation. Control short hairpin RNA had no effect on these TGF-β1-induced responses. TGF-β1-induced phosphorylation of Y256 of N-WASP via activation of small Rho GTPase and focal adhesion kinase mediates TGF-β1-induced paracellular permeability and actin cytoskeleton dynamics. In vivo, compared with controls, N-WASP down-regulation increases survival and prevents lung edema in mice induced by bleomycin exposure-a lung injury model in which TGF-β1 plays a critical role. Our data indicate that N-WASP plays a crucial role in the development of TGF-β1-mediated acute lung injury by promoting pulmonary edema via regulation of actin cytoskeleton dynamics.-Wagener, B. M., Hu, M., Zheng, A., Zhao, X., Che, P., Brandon, A., Anjum, N., Snapper, S., Creighton, J., Guan, J.-L., Han, Q., Cai, G.-Q., Han, X., Pittet, J.-F., Ding, Q. Neuronal Wiskott-Aldrich syndrome protein regulates TGF-β1-mediated lung vascular permeability.
Keywords: FAK; IL-1β; acute lung injury; cytoskeletal dynamics; small Rho GTPases.
© FASEB.
Figures



References
-
- Ware L. B., Matthay M. A. (2000) The acute respiratory distress syndrome. N. Engl. J. Med. 342, 1334–1349 - PubMed
-
- Rubenfeld G. D., Caldwell E., Peabody E., Weaver J., Martin D. P., Neff M., Stern E. J., Hudson L. D. (2005) Incidence and outcomes of acute lung injury. N. Engl. J. Med. 353, 1685–1693 - PubMed
-
- Wagener B. M., Roux J., Carles M., Pittet J. F. (2015) Synergistic inhibition of β2-adrenergic receptor-mediated alveolar epithelial fluid transport by interleukin-8 and transforming growth factor-β. Anesthesiology 122, 1084–1092 - PubMed
-
- Lewis J. F., Jobe A. H. (1993) Surfactant and the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 147, 218–233 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

