Dorso-Lateral Frontal Cortex of the Ferret Encodes Perceptual Difficulty during Visual Discrimination
- PMID: 27025995
- PMCID: PMC4812342
- DOI: 10.1038/srep23568
Dorso-Lateral Frontal Cortex of the Ferret Encodes Perceptual Difficulty during Visual Discrimination
Abstract
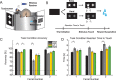
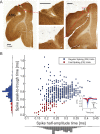
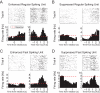
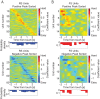
Visual discrimination requires sensory processing followed by a perceptual decision. Despite a growing understanding of visual areas in this behavior, it is unclear what role top-down signals from prefrontal cortex play, in particular as a function of perceptual difficulty. To address this gap, we investigated how neurons in dorso-lateral frontal cortex (dl-FC) of freely-moving ferrets encode task variables in a two-alternative forced choice visual discrimination task with high- and low-contrast visual input. About two-thirds of all recorded neurons in dl-FC were modulated by at least one of the two task variables, task difficulty and target location. More neurons in dl-FC preferred the hard trials; no such preference bias was found for target location. In individual neurons, this preference for specific task types was limited to brief epochs. Finally, optogenetic stimulation confirmed the functional role of the activity in dl-FC before target touch; suppression of activity in pyramidal neurons with the ArchT silencing opsin resulted in a decrease in reaction time to touch the target but not to retrieve reward. In conclusion, dl-FC activity is differentially recruited for high perceptual difficulty in the freely-moving ferret and the resulting signal may provide top-down behavioral inhibition.
Figures
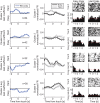
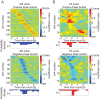
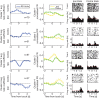
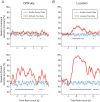
Similar articles
-
Neuronal Adaptation Reveals a Suboptimal Decoding of Orientation Tuned Populations in the Mouse Visual Cortex.J Neurosci. 2019 May 15;39(20):3867-3881. doi: 10.1523/JNEUROSCI.3172-18.2019. Epub 2019 Mar 4. J Neurosci. 2019. PMID: 30833509 Free PMC article.
-
Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search.J Neurophysiol. 1996 Dec;76(6):4040-55. doi: 10.1152/jn.1996.76.6.4040. J Neurophysiol. 1996. PMID: 8985899
-
Speed and accuracy of visual image discrimination by rats.Front Neural Circuits. 2013 Dec 18;7:200. doi: 10.3389/fncir.2013.00200. eCollection 2013. Front Neural Circuits. 2013. PMID: 24385954 Free PMC article.
-
Predicting Perceptual Decisions Using Visual Cortical Population Responses and Choice History.J Neurosci. 2019 Aug 21;39(34):6714-6727. doi: 10.1523/JNEUROSCI.0035-19.2019. Epub 2019 Jun 24. J Neurosci. 2019. PMID: 31235648 Free PMC article.
-
Memory-guided sensory comparisons in the prefrontal cortex: contribution of putative pyramidal cells and interneurons.J Neurosci. 2012 Feb 22;32(8):2747-61. doi: 10.1523/JNEUROSCI.5135-11.2012. J Neurosci. 2012. PMID: 22357858 Free PMC article.
Cited by
-
Resting state network topology of the ferret brain.Neuroimage. 2016 Dec;143:70-81. doi: 10.1016/j.neuroimage.2016.09.003. Epub 2016 Sep 2. Neuroimage. 2016. PMID: 27596024 Free PMC article.
-
A ferret brain slice model of oxygen-glucose deprivation captures regional responses to perinatal injury and treatment associated with specific microglial phenotypes.Bioeng Transl Med. 2021 Nov 23;7(2):e10265. doi: 10.1002/btm2.10265. eCollection 2022 May. Bioeng Transl Med. 2021. PMID: 35600642 Free PMC article.
-
Mesoscopic calcium imaging in a head-unrestrained male non-human primate using a lensless microscope.Nat Commun. 2024 Feb 10;15(1):1271. doi: 10.1038/s41467-024-45417-6. Nat Commun. 2024. PMID: 38341403 Free PMC article.
-
Theta Oscillations Organize Spiking Activity in Higher-Order Visual Thalamus during Sustained Attention.eNeuro. 2018 Apr 3;5(1):ENEURO.0384-17.2018. doi: 10.1523/ENEURO.0384-17.2018. eCollection 2018 Jan-Feb. eNeuro. 2018. PMID: 29619407 Free PMC article.
-
Silencing Neurons: Tools, Applications, and Experimental Constraints.Neuron. 2017 Aug 2;95(3):504-529. doi: 10.1016/j.neuron.2017.06.050. Neuron. 2017. PMID: 28772120 Free PMC article. Review.
References
-
- Fuster J. M. The Prefrontal Cortex. Creativity 1, 410 (2008).
-
- Jacobson S., Butters N. & Tovsky N. J. Afferent and efferent subcortical projections of behaviorally defined sectors of prefrontal granular cortex. Brain Res 159, 279–296 (1978). - PubMed
-
- Pandya D. N. & Kuypers H. G. Cortico-cortical connections in the rhesus monkey. Brain Res 13, 13–36 (1969). - PubMed
-
- Funahashi S., Chafee M. V. & Goldman-Rakic P. S. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature 365, 753–756 (1993). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

