Peripheral, but not central, GLP-1 receptor signaling is required for improvement in glucose tolerance after Roux-en-Y gastric bypass in mice
- PMID: 27026085
- PMCID: PMC4888530
- DOI: 10.1152/ajpendo.00412.2015
Peripheral, but not central, GLP-1 receptor signaling is required for improvement in glucose tolerance after Roux-en-Y gastric bypass in mice
Abstract
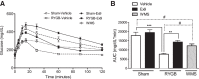
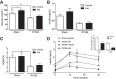
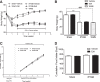
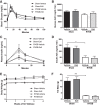
Roux-en-Y gastric bypass (RYGB) causes profound weight loss and remission of diabetes by influencing metabolic physiology, yet the mechanisms behind these clinical improvements remain undefined. After RYGB, levels of glucagon-like peptide-1 (GLP-1), a hormone that enhances insulin secretion and promotes satiation, are substantially elevated. Because GLP-1 signals in both the periphery and the brain to influence energy balance and glucose regulation, we aimed to determine the relative requirements of these systems to weight loss and improved glucose tolerance following RYGB surgery in mice. By pharmacologically blocking peripheral or central GLP-1R signaling, we examined whether GLP-1 action is necessary for the metabolic improvements observed after RYGB. Diet-induced obese mice underwent RYGB or sham operation and were implanted with osmotic pumps delivering the GLP-1R antagonist exendin-(9-39) (2 pmol·kg(-1)·min(-1) peripherally; 0.5 pmol·kg(-1)·min(-1) centrally) for up to 10 wk. Blockade of peripheral GLP-1R signaling partially reversed the improvement in glucose tolerance after RYGB. In contrast, fasting glucose and insulin sensitivity, as well as body weight, were unaffected by GLP-1R antagonism. Central GLP-1R signaling did not appear to be required for any of the metabolic improvements seen after this operation. Collectively, these results suggest a detectable but only modest role for GLP-1 in mediating the effects of RYGB and that this role is limited to its well-described action on glucose regulation.
Keywords: Roux-en-Y gastric bypass; central regulation; glucagon-like peptide-1; glucose tolerance; obesity.
Copyright © 2016 the American Physiological Society.
Figures

References
-
- Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122: 248.e5–256.e5, 2009. - PubMed
-
- Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141: 950–958, 2011. - PMC - PubMed
-
- Gersin KS, Keller JE, Stefanidis D, Simms CS, Abraham DD, Deal SE, Kuwada TS, Heniford BT. Duodenal- jejunal bypass sleeve: a totally endoscopic device for the treatment of morbid obesity. Surg Innov 14: 275–278, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

