Protein oxidation and peroxidation
- PMID: 27026395
- PMCID: PMC4819570
- DOI: 10.1042/BJ20151227
Protein oxidation and peroxidation
Abstract
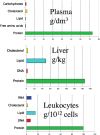
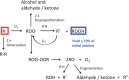
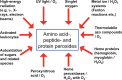
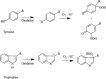
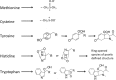
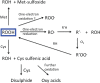
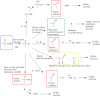
Proteins are major targets for radicals and two-electron oxidants in biological systems due to their abundance and high rate constants for reaction. With highly reactive radicals damage occurs at multiple side-chain and backbone sites. Less reactive species show greater selectivity with regard to the residues targeted and their spatial location. Modification can result in increased side-chain hydrophilicity, side-chain and backbone fragmentation, aggregation via covalent cross-linking or hydrophobic interactions, protein unfolding and altered conformation, altered interactions with biological partners and modified turnover. In the presence of O2, high yields of peroxyl radicals and peroxides (protein peroxidation) are formed; the latter account for up to 70% of the initial oxidant flux. Protein peroxides can oxidize both proteins and other targets. One-electron reduction results in additional radicals and chain reactions with alcohols and carbonyls as major products; the latter are commonly used markers of protein damage. Direct oxidation of cysteine (and less commonly) methionine residues is a major reaction; this is typically faster than with H2O2, and results in altered protein activity and function. Unlike H2O2, which is rapidly removed by protective enzymes, protein peroxides are only slowly removed, and catabolism is a major fate. Although turnover of modified proteins by proteasomal and lysosomal enzymes, and other proteases (e.g. mitochondrial Lon), can be efficient, protein hydroperoxides inhibit these pathways and this may contribute to the accumulation of modified proteins in cells. Available evidence supports an association between protein oxidation and multiple human pathologies, but whether this link is causal remains to be established.
Keywords: UV; amino acid oxidation; hydroperoxides; peroxidation; peroxides; protein oxidation; radicals; singlet oxygen.
© 2016 Authors.
Figures


References
-
- Halliwell B., Gutteridge J.M.C. Free Radicals in Biology & Medicine. Oxford: Oxford University Press; 2015. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

