Multi-walled carbon nanotube induces nitrative DNA damage in human lung epithelial cells via HMGB1-RAGE interaction and Toll-like receptor 9 activation
- PMID: 27026438
- PMCID: PMC4812657
- DOI: 10.1186/s12989-016-0127-7
Multi-walled carbon nanotube induces nitrative DNA damage in human lung epithelial cells via HMGB1-RAGE interaction and Toll-like receptor 9 activation
Abstract
Background: Carbon nanotube (CNT) is used for various industrial purposes, but exhibits carcinogenic effects in experimental animals. Chronic inflammation in the respiratory system may participate in CNT-induced carcinogenesis. 8-Nitroguanine (8-nitroG) is a mutagenic DNA lesion formed during inflammation. We have previously reported that multi-walled CNT (MWCNT) induced 8-nitroG formation in lung epithelial cells and this process involved endocytosis. To clarify the mechanism of CNT-induced carcinogenesis, we examined the role of Toll-like receptor (TLR) 9, which resides in endosomes and lysosomes, in 8-nitroG formation in human lung epithelial cell lines.
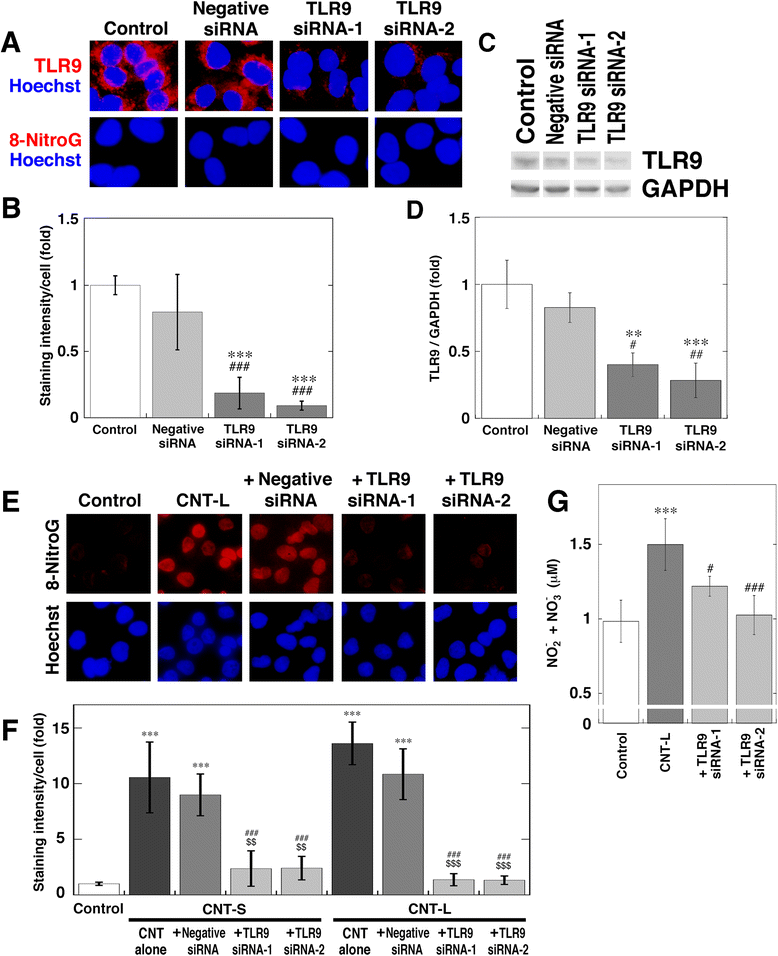
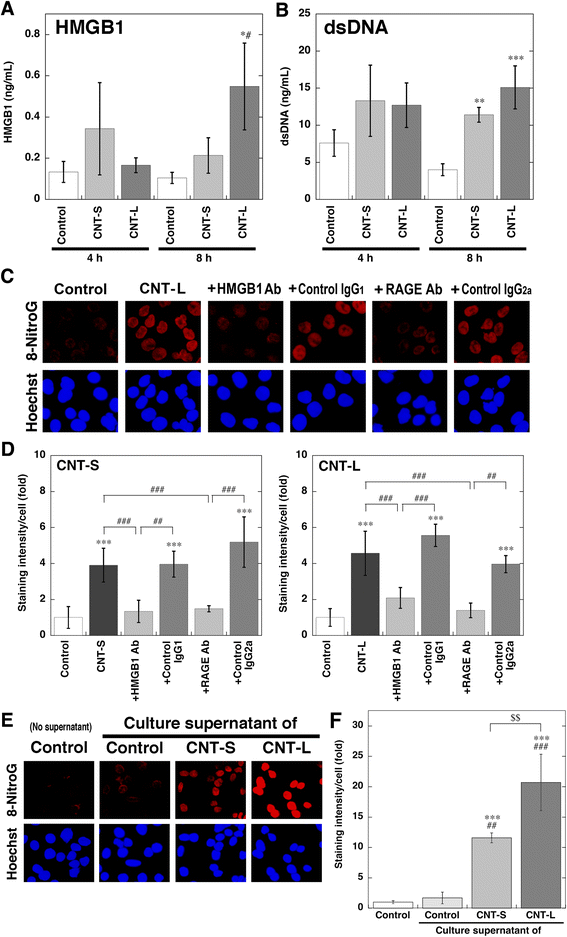
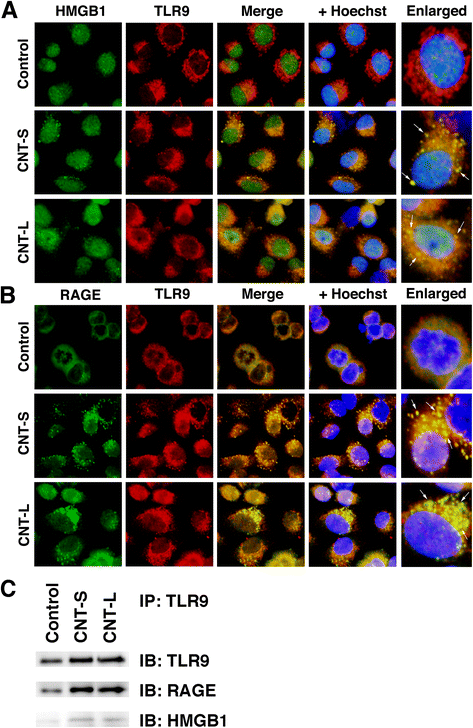
Methods: We performed immunocytochemistry to examine 8-nitroG formation in A549 and HBEpC cells treated with MWCNT with a length of 1-2 μm (CNT-S) or 5-15 μm (CNT-L) and a diameter of 20-40 nm. We examined inhibitory effects of endocytosis inhibitors, small interfering RNA (siRNA) for TLR9, and antibodies against high-mobility group box-1 (HMGB1) and receptor for advanced glycation end-products (RAGE) on 8-nitroG formation. The release of HMGB1 and double-stranded DNA (dsDNA) into the culture supernatant from MWCNT-treated cells was examined by ELISA and fluorometric analysis, respectively. The association of these molecules was examined by double immunofluorescent staining and co-immunoprecipitation.
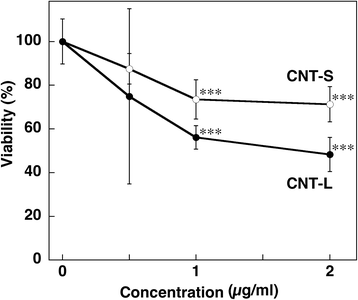
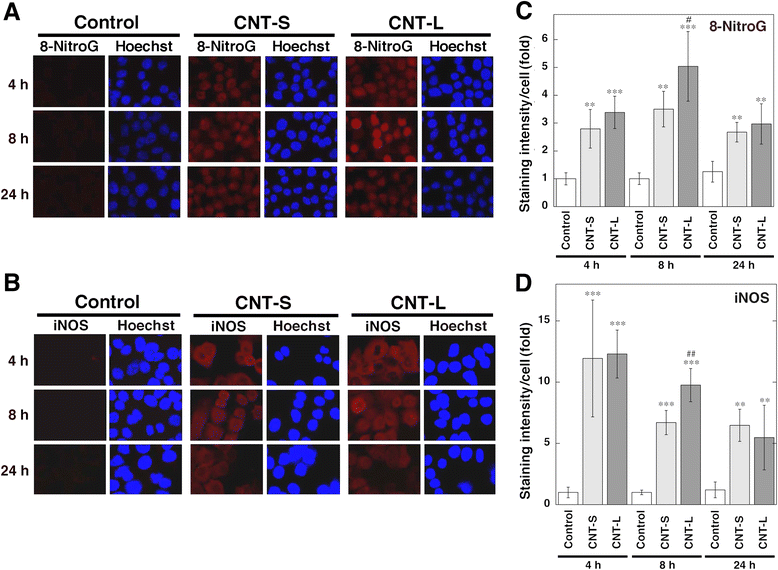
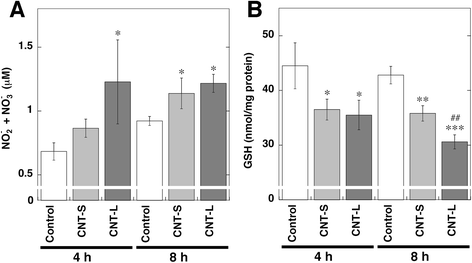
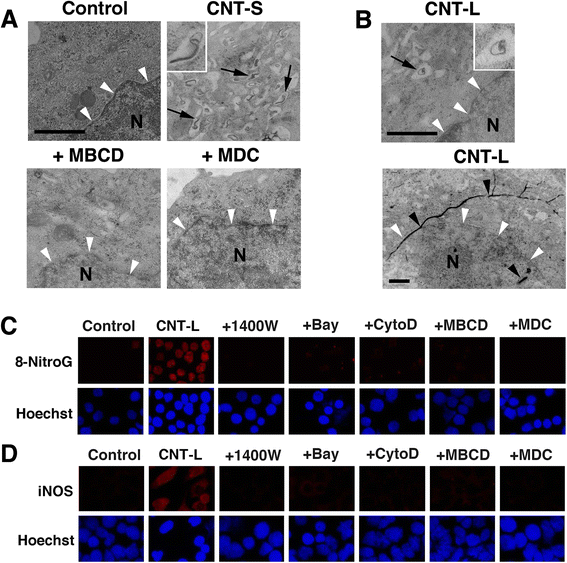
Results: CNT-L significantly increased 8-nitroG formation at 0.05 μg/ml in A549 cells and its intensity reached a maximum at 1 μg/ml. CNT-L tended to induce stronger cytotoxicity and 8-nitroG formation than CNT-S. Endocytosis inhibitors, TLR9 siRNA and antibodies against HMGB1 and RAGE largely reduced MWCNT-induced 8-nitroG formation. MWCNT increased the release of HMGB1 and dsDNA from A549 cells into culture supernatant. The culture supernatant of MWCNT-exposed cells induced 8-nitroG formation in fresh A549 cells. Double immunofluorescent staining and co-immunoprecipitation showed that TLR9 was associated with HMGB1 and RAGE in lysosomes of MWCNT-treated cells.
Conclusions: MWCNT induces injury or necrosis of lung epithelial cells, which release HMGB1 and DNA into the extracellular space. The HMGB1-DNA complex binds to RAGE on neighboring cells and then CpG DNA is recognized by TLR9 in lysosomes, leading to generation of nitric oxide and 8-nitroG formation. This is the first study demonstrating that TLR9 and related molecules participate in MWCNT-induced genotoxicity and may contribute to carcinogenesis.
Keywords: 8-nitroguanine; Carbon nanotube; Carcinogenesis; Cytotoxicity; DNA damage; Endocytosis; High-mobility group box-1, Receptor for advanced glycation end-products, Toll-like receptor; Nitric oxide.
Figures




Similar articles
-
Nitrative DNA damage induced by multi-walled carbon nanotube via endocytosis in human lung epithelial cells.Toxicol Appl Pharmacol. 2012 Apr 15;260(2):183-92. doi: 10.1016/j.taap.2012.02.010. Epub 2012 Feb 21. Toxicol Appl Pharmacol. 2012. PMID: 22373798
-
Nitrative DNA damage in lung epithelial cells exposed to indium nanoparticles and indium ions.Sci Rep. 2020 Jul 1;10(1):10741. doi: 10.1038/s41598-020-67488-3. Sci Rep. 2020. PMID: 32612147 Free PMC article.
-
Nitrative DNA damage in cultured macrophages exposed to indium oxide.J Occup Health. 2018 Mar 27;60(2):148-155. doi: 10.1539/joh.17-0146-OA. Epub 2017 Nov 29. J Occup Health. 2018. PMID: 29187674 Free PMC article.
-
RAGE and TLRs: relatives, friends or neighbours?Mol Immunol. 2013 Dec;56(4):739-44. doi: 10.1016/j.molimm.2013.07.008. Epub 2013 Aug 14. Mol Immunol. 2013. PMID: 23954397 Review.
-
An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies.Pharmacol Ther. 2014 Mar;141(3):347-57. doi: 10.1016/j.pharmthera.2013.11.001. Epub 2013 Nov 9. Pharmacol Ther. 2014. PMID: 24220159 Review.
Cited by
-
MicroRNA expression in lung tissues of asbestos-exposed mice: Upregulation of miR-21 and downregulation of tumor suppressor genes Pdcd4 and Reck.J Occup Health. 2021 Jan;63(1):e12282. doi: 10.1002/1348-9585.12282. J Occup Health. 2021. PMID: 34679210 Free PMC article.
-
Adverse outcome pathways as a tool for the design of testing strategies to support the safety assessment of emerging advanced materials at the nanoscale.Part Fibre Toxicol. 2020 May 25;17(1):16. doi: 10.1186/s12989-020-00344-4. Part Fibre Toxicol. 2020. PMID: 32450889 Free PMC article. Review.
-
The role of high mobility group box 1 protein in acute cerebrovascular diseases.Biomed Rep. 2018 Sep;9(3):191-197. doi: 10.3892/br.2018.1127. Epub 2018 Jul 10. Biomed Rep. 2018. PMID: 30271593 Free PMC article. Review.
-
Recent insights on indirect mechanisms in developmental toxicity of nanomaterials.Part Fibre Toxicol. 2020 Jul 11;17(1):31. doi: 10.1186/s12989-020-00359-x. Part Fibre Toxicol. 2020. PMID: 32653006 Free PMC article. Review.
-
In vitro toxicity of carbon nanotubes: a systematic review.RSC Adv. 2022 May 31;12(25):16235-16256. doi: 10.1039/d2ra02519a. eCollection 2022 May 23. RSC Adv. 2022. PMID: 35733671 Free PMC article. Review.
References
-
- Pacurari M, Yin XJ, Zhao J, Ding M, Leonard SS, Schwegler-Berry D, et al. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-κB, and Akt in normal and malignant human mesothelial cells. Environ Health Perspect. 2008;116:1211–7. doi: 10.1289/ehp.10924. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

