Production of secretory cutinase by recombinant Saccharomyces cerevisiae protoplasts
- PMID: 27026857
- PMCID: PMC4766152
- DOI: 10.1186/s40064-016-1806-4
Production of secretory cutinase by recombinant Saccharomyces cerevisiae protoplasts
Abstract
Background: During heterologous protein production using recombinant microbes, the protein tends to accumulate in the cell and may not be secreted. Here, we studied the production of secretory cutinase (heterologous protein) by recombinant Saccharomyces cerevisiae protoplasts.
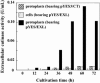
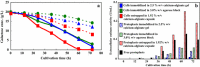
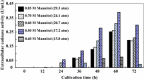
Findings: Recombinant S. cerevisiae cells (i.e., cells into which the cutinase gene was transferred) secreted trace amounts of cutinase into the broth. Approximately 28 % of the cutinase produced in the cells localized to the cell walls and/or between cell wall and cell membrane (CW). In comparison with cell culture, protoplasts in a static culture secreted measurable amounts of cutinase into the broth. Protoplasts were protected from physical and osmotic stresses by entrapping them in a membrane capsule with a low-viscous liquid-core of 1.92 % w/v calcium-alginate. To increase secretory cutinase production, the entrapped protoplasts were cultivated in a shake flask at low osmotic pressure without disruption. During 60 h of cultivation, the extracellular cutinase activity of the free protoplasts at 29.3 atm and protoplasts entrapped in the capsule at 17.2 atm were 0.13 and 0.39 U/mL, respectively.
Conclusions: This is the first report which demonstrates that the efficient production of a secretable enzyme by using protoplasts isolated from recombinant microbes. This system described here is useful to produce products that accumulate in the CW.
Keywords: Cutinase; Immobilization; Protoplast; Recombinant yeast.
Figures
Similar articles
-
Invertase production by Saccharomyces cerevisiae protoplasts immobilized in strontium alginate gel beads.J Biosci Bioeng. 2000;89(5):498-500. doi: 10.1016/s1389-1723(00)89105-7. J Biosci Bioeng. 2000. PMID: 16232786
-
Production of wild-type and peptide fusion cutinases by recombinant Saccharomyces cerevisiae MM01 strains.Biotechnol Bioeng. 2002 Jun 20;78(6):692-8. doi: 10.1002/bit.10252. Biotechnol Bioeng. 2002. PMID: 11992534
-
Production of cell wall accumulative enzymes using immobilized protoplasts of Catharanthus roseus in agarose gel.Biotechnol Lett. 2003 Oct;25(20):1687-93. doi: 10.1023/a:1026025617123. Biotechnol Lett. 2003. PMID: 14626409
-
Electrophysiology in the eukaryotic model cell Saccharomyces cerevisiae.Pflugers Arch. 1998 Nov;436(6):999-1013. doi: 10.1007/s004240050735. Pflugers Arch. 1998. PMID: 9799419 Review.
-
Cutinase: from molecular level to bioprocess development.Biotechnol Bioeng. 1999;66(1):17-34. doi: 10.1002/(sici)1097-0290(1999)66:1<17::aid-bit2>3.0.co;2-f. Biotechnol Bioeng. 1999. PMID: 10556791 Review.
References
-
- Aoyagi H. Application of plant protoplasts for the production of useful metabolites. Biochem Eng J. 2009;56:1–8. doi: 10.1016/j.bej.2010.05.004. - DOI
-
- Calado CRC, Taipa MA, Cabral JMS, Fonseca LP. Optimisation of culture conditions and characterisation of cutinase produced by recombinant Saccharomyces cerevisiae. Enzyme Microb Technol. 2002;31:161–170. doi: 10.1016/S0141-0229(02)00084-4. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

