Endothelial Mechanosignaling: Does One Sensor Fit All?
- PMID: 27027326
- PMCID: PMC5011625
- DOI: 10.1089/ars.2015.6493
Endothelial Mechanosignaling: Does One Sensor Fit All?
Abstract
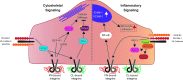
Significance: Forces are important in the cardiovascular system, acting as regulators of vascular physiology and pathology. Residing at the blood vessel interface, cells (endothelial cell, EC) are constantly exposed to vascular forces, including shear stress. Shear stress is the frictional force exerted by blood flow, and its patterns differ based on vessel geometry and type. These patterns range from uniform laminar flow to nonuniform disturbed flow. Although ECs sense and differentially respond to flow patterns unique to their microenvironment, the mechanisms underlying endothelial mechanosensing remain incompletely understood.
Recent advances: A large body of work suggests that ECs possess many mechanosensors that decorate their apical, junctional, and basal surfaces. These potential mechanosensors sense blood flow, translating physical force into biochemical signaling events.
Critical issues: Understanding the mechanisms by which proposed mechanosensors sense and respond to shear stress requires an integrative approach. It is also critical to understand the role of these mechanosensors not only during embryonic development but also in the different vascular beds in the adult. Possible cross talk and integration of mechanosensing via the various mechanosensors remain a challenge.
Future directions: Determination of the hierarchy of endothelial mechanosensors is critical for future work, as is determination of the extent to which mechanosensors work together to achieve force-dependent signaling. The role and primary sensors of shear stress during development also remain an open question. Finally, integrative approaches must be used to determine absolute mechanosensory function of potential mechanosensors. Antioxid. Redox Signal. 25, 373-388.
Figures





References
-
- Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, and Bernfield M. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat Genet 25: 329–332, 2000 - PubMed
-
- Ando J, Ohtsuka A, Korenaga R, Kawamura T, and Kamiya A. Wall shear stress rather than shear rate regulates cytoplasmic Ca++ responses to flow in vascular endothelial cells. Biochem Biophys Res Commun 190: 716–723, 1993 - PubMed
-
- Ando J. and Yamamoto K. Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal 15: 1389–1403, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
