Poly(ADP-ribose) polymerase 1 inhibition protects cardiomyocytes from inflammation and apoptosis in diabetic cardiomyopathy
- PMID: 27027354
- PMCID: PMC5094949
- DOI: 10.18632/oncotarget.8343
Poly(ADP-ribose) polymerase 1 inhibition protects cardiomyocytes from inflammation and apoptosis in diabetic cardiomyopathy
Abstract
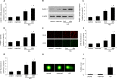
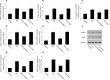
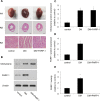
Diabetic cardiomyopathy (DCM) is characterized by structural alterations such as cardiomyocyte hypertrophy, necrosis and focal fibrosis. Poly(ADP-ribose) polymerase 1 (PARP-1) is a nuclear enzyme which can be activated by DNA damage and plays a critical role in various diseases. We hypothesized that PARP-1 may play an important role in DCM and that its inhibition may protect cardiomyocytes from inflammation and apoptosis in DCM. H9c2 cardiomyocytes were treated with normal glucose, mannitol or high glucose (HG). Male C57BL/6 mice or PARP-1-/- mice were treated with streptozotocin (STZ) by intraperitoneal injection for 5 consecutive days to induce diabetes. In vitro, HG stimulation induced oxidative stress and DNA damage and increased PARP-1 expression and activity. Compared with the control, pretreatment with PARP-1 siRNA signiï¬cantly reduced HG-induced inflammatory response, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6 secretion, and intercellular adhesion molecule-1 (ICAM-1) and inducible nitric oxide synthase (iNOS) expression. PARP-1 inhibition reduced HG-induced cardiomyocyte apoptosis through downregulation of cleaved caspases and activation of IGF-1R/Akt pathway. In vivo, hyperglycemia increased the protein expression of nitrotyrosine and PARP-1 as well as PARP-1 activity. PARP-1 gene deletion significantly improved cardiac dysfunction and reduced inflammatory response and apoptosis. This work demonstrated the critical role of PARP-1 in diabetic heart injury, and suggested that PARP-1 inhibition may be a feasible strategy for the treatment of DCM.
Keywords: Pathology Section; apoptosis; diabetic cardiomyopathy; hyperglycemia; inflammatory response; poly(ADP-ribose) polymerase 1.
Conflict of interest statement
No conflict of interest for all authors.
Figures





References
-
- Kanda T, Hayashi K, Wakino S, Homma K, Yoshioka K, Hasegawa K, Sugano N, Tatematsu S, Takamatsu I, Mitsuhashi T, Saruta T. Role of rho-kinase and p27 in angiotensin ii-induced vascular injury. Hypertension. 2005;45:724–729. - PubMed
-
- Westermann D, Van Linthout S, Dhayat S, Dhayat N, Escher F, Bücker-Gärtner C, Spillmann F, Noutsias M, Riad A, Schultheiss HP, Tschöpe C. Cardioprotective and anti-inflammatory effects of interleukin converting enzyme inhibition in experimental diabetic cardiomyopathy. Diabetes. 2007;56:1834–1841. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

