Anticoagulants (extended duration) for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair
- PMID: 27027384
- PMCID: PMC10332795
- DOI: 10.1002/14651858.CD004179.pub2
Anticoagulants (extended duration) for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair
Abstract
Background: The optimal duration of thromboprophylaxis after total hip or knee replacement, or hip fracture repair remains controversial. It is common practice to administer prophylaxis using low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH) until discharge from hospital, usually seven to 14 days after surgery. International guidelines recommend extending thromboprophylaxis for up to 35 days following major orthopaedic surgery but the recommendation is weak due to moderate quality evidence. In addition, recent oral anticoagulants that exert effect by direct inhibition of thrombin or activated factor X lack the need for monitoring and have few known drug interactions. Interest in this topic remains high.
Objectives: To assess the effects of extended-duration anticoagulant thromboprophylaxis for the prevention of venous thromboembolism (VTE) in people undergoing elective hip or knee replacement surgery, or hip fracture repair.
Search methods: The Cochrane Vascular Information Specialist searched the Specialised Register (last searched May 2015) and CENTRAL (2015, Issue 4). Clinical trials databases were searched for ongoing or unpublished studies.
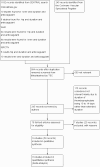
Selection criteria: Randomised controlled trials assessing extended-duration thromboprophylaxis (five to seven weeks) using accepted prophylactic doses of LMWH, UFH, vitamin K antagonists (VKA) or direct oral anticoagulants (DOAC) compared with short-duration thromboprophylaxis (seven to 14 days) followed by placebo, no treatment or similar extended-duration thromboprophylaxis with LMWH, UFH, VKA or DOACs in participants undergoing hip or knee replacement or hip fracture repair.
Data collection and analysis: We independently selected trials and extracted data. Disagreements were resolved by discussion. We performed fixed-effect model meta-analyses with odds ratios (ORs) and 95% confidence intervals (CIs). We used a random-effects model when there was heterogeneity.
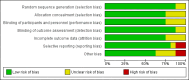
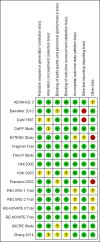
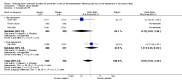
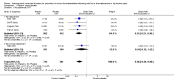
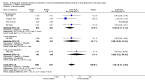
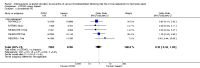
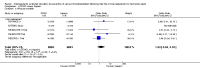
Main results: We included 16 studies (24,930 participants); six compared heparin with placebo, one compared VKA with placebo, two compared DOAC with placebo, one compared VKA with heparin, five compared DOAC with heparin and one compared anticoagulants chosen at investigators' discretion with placebo. Three trials included participants undergoing knee replacement. No studies assessed hip fracture repair.Trials were generally of good methodological quality. The main reason for unclear risk of bias was insufficient reporting. The quality of evidence according to GRADE was generally moderate, as some comparisons included a single study, low number of events or heterogeneity between studies leading to wide CIs.We showed no difference between extended-duration heparin and placebo in symptomatic VTE (OR 0.59, 95% CI 0.35 to 1.01; 2329 participants; 5 studies; high quality evidence), symptomatic deep vein thrombosis (DVT) (OR 0.73, 95% CI 0.39 to 1.38; 2019 participants; 4 studies; moderate quality evidence), symptomatic pulmonary embolism (PE) (OR 0.61, 95% CI 0.16 to 2.33; 1595 participants; 3 studies; low quality evidence) and major bleeding (OR 0.59, 95% CI 0.14 to 2.46; 2500 participants; 5 studies; moderate quality evidence). Minor bleeding was increased in the heparin group (OR 2.01, 95% CI 1.43 to 2.81; 2500 participants; 5 studies; high quality evidence). Clinically relevant non-major bleeding was not reported.We showed no difference between extended-duration VKA and placebo (one study, 360 participants) for symptomatic VTE (OR 0.10, 95% CI 0.01 to 1.94; moderate quality evidence), symptomatic DVT (OR 0.13, 95% CI 0.01 to 2.62; moderate quality evidence), symptomatic PE (OR 0.32, 95% CI 0.01 to 7.84; moderate quality evidence) and major bleeding (OR 2.89, 95% CI 0.12 to 71.31; low quality evidence). Clinically relevant non-major bleeding and minor bleeding were not reported.Extended-duration DOAC showed reduced symptomatic VTE (OR 0.20, 95% CI 0.06 to 0.68; 2419 participants; 1 study; moderate quality evidence) and symptomatic DVT (OR 0.18, 95% CI 0.04 to 0.81; 2459 participants; 2 studies; high quality evidence) compared to placebo. No differences were found for symptomatic PE (OR 0.25, 95% CI 0.03 to 2.25; 1733 participants; 1 study; low quality evidence), major bleeding (OR 1.00, 95% CI 0.06 to 16.02; 2457 participants; 1 study; low quality evidence), clinically relevant non-major bleeding (OR 1.22, 95% CI 0.76 to 1.95; 2457 participants; 1 study; moderate quality evidence) and minor bleeding (OR 1.18, 95% CI 0.74 to 1.88; 2457 participants; 1 study; moderate quality evidence).We showed no difference between extended-duration anticoagulants chosen at investigators' discretion and placebo (one study, 557 participants, low quality evidence) for symptomatic VTE (OR 0.50, 95% CI 0.09 to 2.74), symptomatic DVT (OR 0.33, 95% CI 0.03 to 3.21), symptomatic PE (OR 1.00, 95% CI 0.06 to 16.13), and major bleeding (OR 5.05, 95% CI 0.24 to 105.76). Clinically relevant non-major bleeding and minor bleeding were not reported.We showed no difference between extended-duration VKA and heparin (one study, low quality evidence) for symptomatic VTE (OR 1.64, 95% CI 0.85 to 3.16; 1279 participants), symptomatic DVT (OR 1.36, 95% CI 0.69 to 2.68; 1279 participants), symptomatic PE (OR 9.16, 95% CI 0.49 to 170.42; 1279 participants), major bleeding (OR 3.87, 95% CI 1.91 to 7.85; 1272 participants) and minor bleeding (OR 1.33, 95% CI 0.64 to 2.76; 1279 participants). Clinically relevant non-major bleeding was not reported.We showed no difference between extended-duration DOAC and heparin for symptomatic VTE (OR 0.70, 95% CI 0.28 to 1.70; 15,977 participants; 5 studies; low quality evidence), symptomatic DVT (OR 0.60, 95% CI 0.11 to 3.27; 15,977 participants; 5 studies; low quality evidence), symptomatic PE (OR 0.91, 95% CI 0.43 to 1.94; 14,731 participants; 5 studies; moderate quality evidence), major bleeding (OR 1.11, 95% CI 0.79 to 1.54; 16,199 participants; 5 studies; high quality evidence), clinically relevant non-major bleeding (OR 1.08, 95% CI 0.90 to 1.28; 15,241 participants; 4 studies; high quality evidence) and minor bleeding (OR 0.95, 95% CI 0.82 to 1.10; 11,766 participants; 4 studies; high quality evidence).
Authors' conclusions: Moderate quality evidence suggests extended-duration anticoagulants to prevent VTE should be considered for people undergoing hip replacement surgery, although the benefit should be weighed against the increased risk of minor bleeding. Further studies are needed to better understand the association between VTE and extended-duration oral anticoagulants in relation to knee replacement and hip fracture repair, as well as outcomes such as distal and proximal DVT, reoperation, wound infection and healing.
Conflict of interest statement
RF: none known. MS: none known. MS is a member of Cochrane Vascular's editorial staff. To prevent any conflict of interest issues editorial decisions and activities related to this review were carried out by other editorial staff where appropriate.
Figures





















































Update of
References
References to studies included in this review
ADVANCE 3 {published data only}
-
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. New England Journal of Medicine 2010;363(26):2487‐98. - PubMed
-
- NCT00423319. Study of an investigational drug for the prevention of thrombosis‐related events following hip replacement surgery (ADVANCE‐3). https://clinicaltrials.gov/ct2/show/NCT00423319?term=NCT00423319&rank=1 (accessed 30 August 2015).
-
- Pineo GF, Gallus AS, Raskob GE, Chen D, Ramirez L‐M, Ramacciotti E, et al. Apixaban after hip or knee arthroplasty versus enoxaparin: Efficacy and safety in key clinical subgroups. Journal of Thrombosis and Haemostasis 2013;11(3):444‐51. - PubMed
-
- Raskob GE, Gallus AS, Pineo GF, Chen D, Ramirez LM, Lassen MR. Apixaban versus enoxaparin for thromboprophylaxis after joint replacement surgery: pooled analysis of major venous thromboembolism and bleeding in 8,464 patients from the advance 2 and 3 trials [Abstract No. 192]. Blood. 2010; Vol. 116. - PubMed
-
- Raskob GE, Gallus AS, Pineo GF, Chen D, Ramirez LM, Wright RT, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip or knee replacement: pooled analysis of major venous thromboembolism and bleeding in 8464 patients from the ADVANCE‐2 and ADVANCE‐3 trials. Journal of Bone and Joint Surgery. British Volume 2012;94(2):257‐64. - PubMed
Barrellier 2010 {published data only}
-
- Barrellier MT, Lebel B, Parienti JJ, Mismetti P, Dutheil JJ, Vielpeau C, et al. Short versus extended thromboprophylaxis after total knee arthroplasty: a randomized comparison. Thrombosis Research 2010;126(4):e298‐304. - PubMed
Dahl 1997 {published data only}
-
- Anon. Dalteparin new treatment duration: new dosage. Is prolonged prophylaxis needed?. Prescrire International 2000;9(45):197‐98. - PubMed
-
- Arnesen H, Dahl OE, Aspelin T, Seljeflot I, Kierulf P, Lyberg T. Sustained prothrombotic profile after hip replacement surgery: the influence of prolonged prophylaxis with dalteparin. Journal of Thrombosis and Haemostasis 2003;1(5):971‐5. - PubMed
-
- Dahl OE, Andreassen G, Aspelin T, Muller C, Mathiesen P, Nyhus S, et al. Prolonged thromboprophylaxis following hip replacement surgery‐‐results of a double‐blind, prospective, randomised, placebo‐controlled study with dalteparin (Fragmin). Thrombosis and Haemostasis 1997;77(1):26‐31. - PubMed
-
- Dahl OE, Andreassen G, Muller C, Mathisen P, Nyhus S, Aspelin T, et al. The effect of prolonged thromboprophylaxis with dalteparin on the frequency of deep vein thrombosis (DVT) and pulmonary embolism (PE) 35 days after hip replacement surgery (HRS). Thrombosis and Haemostasis 1995;73(6):1094‐Abstract No 743.
DaPP Study {published data only}
-
- Lassen MR, Borris LC. Prolonged thromboprophylaxis with low molecular weight heparin (Fragmin) after elective total hip arthroplasty ‐ a placebo controlled study. Thrombosis and Haemostasis. 1995; Vol. 73, issue 6:1104.
-
- Lassen MR, Borris LC, Anderson BS, Jensen HP, Skejo Bro HP, Andersen G, et al. Efficacy and safety of prolonged thromboprophylaxis with a low molecular weight heparin (dalteparin) after total hip arthroplasty‐‐the Danish Prolonged Prophylaxis (DaPP) Study. Thrombosis Research 1998;89(6):281‐7. - PubMed
EXTEND Study {published data only}
-
- Agnelli G, Eriksson BI, Cohen AT, Bergqvist D, Dahl OE, Lassen MR, et al. Safety assessment of new antithrombotic agents: Lessons from the EXTEND study on ximelagatran. Thrombosis Research 2009;123(3):488‐94. - PubMed
Fragmin Trial {published data only}
-
- Hull RD. New insights into extended prophylaxis after orthopaedic surgery ‐ the North American Fragmin Trial experience. Haemostasis. 2000; Vol. 30, issue Suppl 2:95‐100. - PubMed
-
- Hull RD, Pineo GF, Francis C, Bergqvist, D, Fellenius C, Soderberg K, et al. Low‐molecular‐weight heparin prophylaxis using dalteparin extended out‐of‐hospital vs in‐hospital warfarin/out‐of‐hospital placebo in hip arthroplasty patients: a double‐blind, randomized comparison. North American Fragmin Trial Investigators. Archives of Internal Medicine 2000;160(14):2208‐15. - PubMed
French Study {published data only}
-
- Planes A, Vochelle N. The post‐hospital discharge venous thrombosis risk of the orthopedic patient. Orthopedics 1997;20(2 Suppl):18‐21. - PubMed
-
- Planes A, Vochelle N, Compan D, Weisslinger N, Huet Y. Efficacy and safety of prolonged administration of enoxaparin in the prevention of deep venous thrombosis after elective total hip replacement. Orthopaedic Transactions. 1996; Vol. 20, issue 1:274. - PubMed
-
- Planes A, Vochelle N, Darmon J‐Y, Fagola M, Bellaud M, Compan D, et al. Efficacy and safety of postdischarge administration of enoxaparin in the prevention of deep venous thrombosis after total hip replacement. A prospective randomised double‐blind placebo‐controlled trial. Drugs 1996;52(Suppl 7):47‐54. - PubMed
-
- Planes A, Vochelle N, Darmon J‐Y, Fagola M, Bellaud M, Huet Y. Risk of deep‐venous thrombosis after hospital discharge in patients having undergone total hip replacement: double‐blind randomised comparison of enoxaparin versus placebo. Lancet 1996;348(9022):224‐8. - PubMed
-
- Planes A, Vochelle N, Darmon JY. Out‐of‐hospital prophylaxis with low‐molecular‐weight heparin in hip surgery: the French study‐‐venographic outcome at 35 days. Chest 1998;114(2 Suppl Evidence):125S‐9S. - PubMed
Heit 2000 {published data only}
-
- Heit JA, Elliott CG, Trowbridge AA, Hirsh J, Gent M. Extended out‐of‐hospital LMWH venous thromboembolism prophylaxis after total hip or knee replacement surgery. Blood 1998;92:500a. - PubMed
-
- Heit JA, Elliott CG, Trowbridge AA, Morrey BF, Gent M, Hirsh J. Ardeparin sodium for extended out‐of‐hospital prophylaxis against venous thromboembolism after total hip or knee replacement. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 2000;132(11):853‐61. - PubMed
Kolb 2003 {published data only}
-
- Kolb G, Bodamer I, Galster H, Grambach K, Koudela K, Eisele RR, et al. Prolonged prophylaxis with the low molecular weight heparin certoparin reduces the rate of venous thromboembolism after orthopedic surgery. Journal of Thrombosis and Haemostasis. 2003; Vol. Suppl:Abstract: P1868. - PubMed
-
- Kolb G, Bodamer I, Galster H, Seidlmayer C, Grambach K, Koudela K, et al. Reduction of venous thromboembolism following prolonged prophylaxis with the low molecular weight heparin Certoparin after endoprothetic joint replacement or osteosynthesis of the lower limb in elderly patients. Thrombosis and Haemostasis 2003;90(6):1100‐5. - PubMed
Prandoni 2002 {published data only}
-
- Prandoni P, Bruchi O, Sabbion P, Tanduo C, Scudeller A, Sardella C, et al. Prolonged thromboprophylaxis with oral anticoagulants after total hip arthroplasty: a prospective controlled randomized study. Archives of Internal Medicine 2002;162(17):1966‐71. - PubMed
RECORD 1 Trial {published data only}
-
- Anon. Prevention of thromboembolism after knee replacement surgery: rivaroxaban one tablet/once daily superior to twice daily injectable enoxaparin in preventing venous blood clots after total knee replacement surgery in pivotal phase III trial. http://www.bayer.ca/ 2008.
-
- Cohn DM, Hermanides J, Devries JH, Kamphuisen PW, Kuhls S, Homering M, et al. Stress‐induced hyperglycaemia and venous thromboembolism following total hip or total knee arthroplasty. Analysis from the RECORD trials. Thrombosis and Haemostasis 2012;107(2):225‐231. - PubMed
-
- Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. Oral rivaroxaban compared with subcutaneous enoxaparin for extended thromboprophylaxis after total hip arthroplasty: The RECORD1 trial. Blood (ASH Annual Meeting Abstracts) 2007;110(11):6.
-
- Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. Oral rivaroxaban versus subcutaneous enoxaparin for extended thromboprophylaxis after total hip replacement: RECORD1 (abstract 222). British Journal of Haematology 2008;141(Suppl 1):82.
-
- Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. New England Journal of Medicine 2008;358(26):2765‐75. - PubMed
RECORD 2 Trial {published data only}
-
- Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Bandel TJ, et al. A phase III study of extended thromboprophylaxis with oral rivaroxaban versus short‐term subcutaneous enoxaparin after total hip replacement: RECORD2 (abstract O59). Pathophysiology of Haemostasis and Thrombosis 2008;36(Suppl 1):A15.
-
- Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, et al. Extended duration rivaroxaban versus short‐term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double‐blind, randomised controlled trial. Lancet 2008;372(9632):31‐9. - PubMed
-
- Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, et al. Extended thromboprophylaxis with rivaroxaban compared with short‐term thromboprophylaxis with enoxaparin after total hip arthroplasty: The RECORD2 trial. Blood (ASH Annual Meeting Abstracts) 2007;110(11):307.
-
- Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, et al. RECORD2: extended thromboprophylaxis with rivaroxaban versus short‐term thromboprophylaxis with enoxaparin after total hip replacement (abstract 176). British Journal of Haematology 2008;141(Suppl 1):65.
-
- Mouret P, Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Muntz J, et al. Extended thrombosis prophylaxis with rivaroxaban in comparison to short‐term thrombosis prophylaxis with enoxaparin after total hip replacement: The RECORD2 study. Medizinische Klinik 2008;103(3):15.
RE‐NOVATE II Trial {published data only}
-
- Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE‐NOVATE II): A randomised, double‐blind, non‐inferiority trial. Thrombosis and Haemostasis 2011;105(4):721‐9. - PubMed
-
- Huo M, Eriksson B, Dahl O, Kurth A, Hantel S, Hermasson K, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty: the RE‐NOVATE II randomised trial [Abstract No. 0564]. Haematologica 2010; Vol. 92:233.
-
- NCT00657150. Dabigatran etexilate compared with enoxaparin in prevention of venous thromboembolism (VTE) following total hip arthroplasty. https://clinicaltrials.gov/ct2/show/NCT00657150?term=RE‐NOVATE&rank=1 (accessed 30 August 2015).
RE‐NOVATE Trial {published data only}
-
- Eriksson BI, Dahl OE, Rosencher N, Kurth AA, Dijk CN, Frostick SP, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double‐blind, non‐inferiority trial. Lancet 2007;370(9591):949‐56. - PubMed
-
- Eriksson BI, Dahl OE, Rosencher N, Kurth AA, Dijk N, Frostick SP, et al. Dabigatran etexilate is effective and safe for the extended prevention of venous thromboembolism following total hip replacement (abstract OW‐049). Journal of Thrombosis and Haemostasis 2007;5(Suppl 2):OW‐049. - PubMed
-
- NCT00168818. Dabigatran etexilate in extended VTE prevention after hip replacement surgery. https://clinicaltrials.gov/ct2/show/NCT00168818?term=RE‐NOVATE&rank=2 (accessed 30 August 2015).
SACRE Study {published data only}
-
- Samama CM, Vray M, Barre J, Fiessinger JN, Rosencher N, Lecompte T, et al. Extended venous thromboembolism prophylaxis after total hip replacement: a comparison of low‐molecular‐weight heparin with oral anticoagulant. Archives of Internal Medicine 2002;162(19):2191‐6. - PubMed
Zhang 2014 {published data only}
-
- Zhang H, Lin J, Li H, Guan Z, Zhou D, Kon B, et al. Effects of thromboprophylaxis duration on coagulation indicators after total hip replacement. National Medical Journal of China 2014;94(7):525‐8. - PubMed
References to studies excluded from this review
Comp 2001 {published data only}
-
- Comp PC, Spiro TE, Friedman RJ, Whitsett TL, Johnson GJ, Gardiner GA, et al. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement. Enoxaparin Clinical Trial Group. Journal of Bone & Joint Surgery ‐ American Volume 2001;83‐A(3):336‐45. - PubMed
-
- Spiro TE. A double‐blind multicenter clinical trial comparing long term enoxaparin and placebo treatments in the prevention of venous thromboembolic disease after hip and knee replacement surgery. Blood. 1997; Vol. 90, issue Suppl 1:295a.
EPCAT II {published data only}
-
- NCT01720108. Extended venous thromboembolism prophylaxis comparing rivaroxaban to aspirin following total hip and knee arthroplasty (EPCAT II). https://clinicaltrials.gov/ct2/show/NCT01720108 (accessed 30 August 2015).
Kristensen 1990 {published data only}
-
- Kristensen SS, Pedersen P, Pedersen NW, Schmidt SA, Kjaersgaard‐Andersen P. Combined treatment with indomethacin and low‐dose heparin after total hip replacement. Journal of Bone and Joint Surgery. American Volume 1990;72:447‐9. - PubMed
Manganelli 1998 {published data only}
-
- Manganelli D, Pazzagli M, Mazzantini D, Punzi G, Manca M, Vignali C, et al. Prolonged prophylaxis with unfractioned heparin is effective to reduce delayed deep vein thrombosis in total hip replacement. Respiration 1998;65(5):369‐74. - PubMed
-
- Palla A, Manganelli D, Rossi G. Prolonged prophylaxis with unfractioned heparin (UH) is effective to reduce delayed deep vein thrombosis (DVT) in total hip replacement (THR). European Respiratory Journal. 1997; Vol. 10, issue Suppl 25:5S. - PubMed
NPHDO Study Group {published data only}
-
- Haentjens P, Delince P, The Belgian Nadroparin Post‐Hospital Discharge In Orthopedics (NPHDO) Study Group. Prevention of venous thromboembolism after hospital discharge. Continued pharmacologic prophylaxis versus no prophylaxis in patients undergoing total hip replacement. Hip International 2001;11(1):25‐36.
-
- Haentjens P, The Belgian Nadroparin Post‐Hospital Discharge in Orthopedics (NPHDO) Study Group. Post‐hospital discharge prevention of deep vein thrombosis with nadroparin calcium after elective total hip replacement. British Journal of Anaesthesia 1999;82(Suppl 1):78.
-
- The Belgian Nadroparin Post Hospital Discharge in Orthopedics (NPHDO) Study Group. Post hospital discharge prevention of deep vein thrombosis with nadroparin calcium after total hip arthroplasty. Haemostasis. 1998; Vol. 28, issue Suppl 2:292.
PENTHIFRA PLUS Study {published data only}
-
- Anon. A multicenter, multinational, randomized, double‐blind study of fondaparinux sodium (Org31540/SR90107A) versus placebo for the prolonged prevention of VTE in hip fracture surgery. (PENTIHFRA PLUS). Study No: EFC4582. GlaxoSmithKline Clinical Trial Register 2005.
-
- Bauer KA, Eriksson BI, Lassen MR, Turpie AGG. No episode of thrombocytopenia after four‐week administration of fondaparinux, a new synthetic and selective inhibitor of factor Xa, in the PENTHIFRA‐PLUS study. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):P2050.
-
- Dobesh PP. Novel concepts: emerging data and the role of extended prophylaxis following hip fracture surgery. American Journal of Health‐System Pharmacy 2003;60(22 Suppl 7):S15‐9. - PubMed
-
- Eriksson BI. A multicenter, randomized, placebo‐controlled, double‐blind study of fondaparinux for the prolonged prevention of venous thromboembolism in hip fracture surgery. Abstracts of the Sicot/Sirot XXII World Congress 2002:318.
-
- Eriksson BI, Lassen MR. Consistency of efficacy of extended thromboprophylaxis with fondaparinux (arixtra) in prevention of venous thromboembolism (VTE) after hip fracture surgery according to different composite efficacy endpoints: the PENTHIFRA‐PLUS study. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):Abstract P2064.
Swedish Study {published data only}
-
- Bergqvist D, Benoni G, Bjorgell O, Fredin H, Hedlundh U, Nicolas S, et al. Low‐molecular‐weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. New England Journal of Medicine 1996;335(10):696‐700. - PubMed
-
- Bergqvist D, Jönsson B. Cost‐effectiveness of prolonged out‐of‐hospital prophylaxis with low‐molecular‐weight heparin following total hip replacement. Haemostasis 2000;30 Suppl 2:130‐5. - PubMed
-
- Nilsson PE, Bergqvist D, Benoni G, Bjorgell O, Fredin H, Hedlund U, et al. The post‐discharge prophylactic management of the orthopedic patient with low‐molecular‐weight heparin: enoxaparin. Orthopedics 1997;20 Suppl:22‐5. - PubMed
Additional references
Atkins 2004
Cushman 2007
Dotzel 2002
-
- Dotzel, MM. Determination that ardeparin sodium injection was not withdrawn from sale for reasons of safety or effectiveness. US Food and Drug Administration 2002:http://www.fda.gov/ohrms/dockets/98fr/052302b.htm (date accessed September 2015).
Egger 1997
Eikelboom 2001
-
- Eikelboom JW, Quinlan DJ, Douketis JD. Extended‐duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta‐analysis of the randomised trials. Lancet 2001;358(9275):9‐15. - PubMed
Eriksson 1997
-
- Eriksson BI, Wille‐Jorgensen P, Kalebo P, Mouret P, Rosencher N, Bosch P, et al. A comparison of recombinant hirudin with a low‐molecular‐weight heparin to prevent thromboembolic complications after total hip replacement [see comments]. New England Journal of Medicine 1997;337(19):1329‐35. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc). Available from www.gradepro.org. GRADEpro Guideline Development Tool (GRADEpro GDT). McMaster University (developed by Evidence Prime, Inc). Available from www.gradepro.org, 2015.
Guyatt 2012
-
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):7S‐47S. [DOI: 10.1378/chest.1412S3] - DOI - PMC - PubMed
Heit 2015
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirsh 1998
-
- Hirsh J. Evidence for the needs of out‐of‐hospital thrombosis prophylaxis: introduction. Chest 1998;114(2 Suppl Evidence):113S‐4S. - PubMed
Lau 1998
-
- Lau J, Ioannidis JP, Schmid CH. Summing up evidence: one answer is not always enough. Lancet 1998;351(9096):123‐7. - PubMed
Leclerc 1998
-
- Leclerc JR, Gent M, Hirsh J, Geerts WH, Ginsberg JS. The incidence of symptomatic venous thromboembolism after enoxaparin prophylaxis in lower extremity arthroplasty: a cohort study of 1,984 patients. Chest 1998;114(2 Suppl II):115S‐8S. - PubMed
Liew 2014
-
- Liew A. Extended‐duration new oral anticoagulants for venous thromboprophylaxis in patients undergoing total hip arthroplasty: A meta‐analysis of the randomized controlled trials. Journal of Thrombosis and Haemostasis 2014;12(1):107‐9. - PubMed
Mohr 1993
-
- Mohr DN, Silverstein MD, Murtaugh PA, Harrison JM. Prophylactic agents for venous thrombosis in elective hip surgery: Meta‐analysis of studies using venographic assessment. Archives of Internal Medicine 1993;153(19):2221‐8. - PubMed
NICE 2010
-
- National Clinical Guideline Centre. Venous thromboembolism: reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. Methods evidence and guidance. http://www.nice.org.uk/guidance/cg92/evidence/cg92‐venous‐thromboembolis... (accessed September 2015).
NICE 2012
-
- National Clinical Guideline Centre. Venous thromboembolic diseases: the management of venous thromboembolic diseases and the role of thrombophilia testing. Clinical Guideline. Methods, evidence and recommendations. http://www.nice.org.uk/guidance/cg144/evidence/cg144‐venous‐thromboembol... (accessed September 2015).
Nurmohamed 1992
-
- Nurmohamed MT, Rosendaal FR, Buller HR, Dekker E, Hommes DW, Vandenbroucke JP, et al. Low‐molecular‐weight heparin versus standard heparin in general and orthopaedic surgery: a meta‐analysis. Lancet 1992;340(8812):152‐6. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 1997
-
- Robinson KS, Anderson DR, Gross M, Petrie D, Leighton R, Stanish W, et al. Ultrasonographic screening before hospital discharge for deep venous thrombosis after arthroplasty: the post‐arthroplasty screening study. A randomized, controlled trial. Annals of Internal Medicine 1997;127(6):439‐45. - PubMed
SIGN 2010
-
- Scottish Intercollegiate Guidelines Network. Thrombosis Guidelines Prevention and management of venous thromboembolism. A national clinical guideline. http://www.sign.ac.uk/pdf/sign122.pdf (accessed September 2015).
Thompson 1994
Wang 2014
White 2003
-
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107:I‐4–I‐8. - PubMed
References to other published versions of this review
Quinlan 2002
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

