The ever-expanding role of HIF in tumour and stromal biology
- PMID: 27027486
- PMCID: PMC4898054
- DOI: 10.1038/ncb3330
The ever-expanding role of HIF in tumour and stromal biology
Abstract
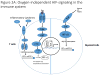
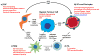
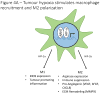
Low oxygen tension (hypoxia) is a hallmark of cancer that influences cancer cell function, but is also an important component of the tumour microenvironment as it alters the extracellular matrix, modulates the tumour immune response and increases angiogenesis. Here we discuss the regulation and role of hypoxia and its key transcriptional mediators, the hypoxia-inducible factor (HIF) family of transcription factors, in the tumour microenvironment and stromal compartments.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

