Silencing of Prrx1b suppresses cellular proliferation, migration, invasion and epithelial-mesenchymal transition in triple-negative breast cancer
- PMID: 27027510
- PMCID: PMC4988287
- DOI: 10.1111/jcmm.12856
Silencing of Prrx1b suppresses cellular proliferation, migration, invasion and epithelial-mesenchymal transition in triple-negative breast cancer
Retraction in
-
Retraction: Silencing of Prrx1b suppresses cellular proliferation, migration, invasion and epithelial-mesenchymal transition in triple-negative breast cancer.J Cell Mol Med. 2024 Apr;28(8):e18312. doi: 10.1111/jcmm.18312. J Cell Mol Med. 2024. PMID: 38661448 Free PMC article.
Abstract
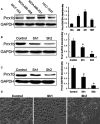
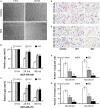
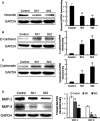
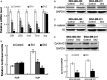
Triple-negative breast cancer (TNBC) is a highly aggressive tumour subtype associated with poor prognosis. The mechanisms involved in TNBC progression remains largely unknown. To date, there are no effective therapeutic targets for this tumour subtype. Paired-related homeobox 1b (Prrx1b), one of major isoforms of Prrx1, has been identified as a new epithelial-mesenchymal transition (EMT) inducer. However, the function of Prrx1b in TNBC has not been elucidated. In this study, we found that Prrx1b was significantly up-regulated in TNBC and associated with tumour size and vascular invasion of breast cancer. Silencing of Prrx1b suppressed the proliferation, migration and invasion of basal-like cancer cells. Moreover, silencing of Prrx1b prevented Wnt/β-catenin signaling pathway and induced the mesenchymal-epithelial transition (MET). Taken together, our data indicated that Prrx1b may be an important regulator of EMT in TNBC cells and a new therapeutic target for interventions against TNBC invasion and metastasis.
Keywords: epithelial-mesenchymal transition; invasion; paired-related homeobox 1b; proliferation; triple-negative breast cancer.
© 2016 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures


Similar articles
-
MiR-212-5p Suppresses the Epithelial-Mesenchymal Transition in Triple-Negative Breast Cancer by Targeting Prrx2.Cell Physiol Biochem. 2017;44(5):1785-1795. doi: 10.1159/000485785. Epub 2017 Dec 6. Cell Physiol Biochem. 2017. PMID: 29216628
-
Profilin 2 (PFN2) promotes the proliferation, migration, invasion and epithelial-to-mesenchymal transition of triple negative breast cancer cells.Breast Cancer. 2021 Mar;28(2):368-378. doi: 10.1007/s12282-020-01169-x. Epub 2020 Oct 12. Breast Cancer. 2021. PMID: 33047272
-
AQP5 promotes epithelial-mesenchymal transition and tumor growth through activating the Wnt/β-catenin pathway in triple-negative breast cancer.Mutat Res. 2024 Jul-Dec;829:111868. doi: 10.1016/j.mrfmmm.2024.111868. Epub 2024 Jun 12. Mutat Res. 2024. PMID: 38959561
-
MDIG in Breast Cancer Progression and Metastasis.Adv Exp Med Biol. 2024;1465:1-14. doi: 10.1007/978-3-031-66686-5_1. Adv Exp Med Biol. 2024. PMID: 39586990 Review.
-
A comprehensive overview of metaplastic breast cancer: Features and treatments.Cancer Sci. 2024 Aug;115(8):2506-2514. doi: 10.1111/cas.16208. Epub 2024 May 12. Cancer Sci. 2024. PMID: 38735837 Free PMC article. Review.
Cited by
-
From Proteomic Mapping to Invasion-Metastasis-Cascade Systemic Biomarkering and Targeted Drugging of Mutant BRAF-Dependent Human Cutaneous Melanomagenesis.Cancers (Basel). 2021 Apr 22;13(9):2024. doi: 10.3390/cancers13092024. Cancers (Basel). 2021. PMID: 33922182 Free PMC article.
-
The rs7911488-T allele promotes the growth and metastasis of colorectal cancer through modulating miR-1307/PRRX1.Cell Death Dis. 2020 Aug 7;11(8):651. doi: 10.1038/s41419-020-02834-x. Cell Death Dis. 2020. PMID: 32811812 Free PMC article.
-
Mapping Intellectual Structures and Research Hotspots of Triple Negative Breast Cancer: A Bibliometric Analysis.Front Oncol. 2022 Jan 3;11:689553. doi: 10.3389/fonc.2021.689553. eCollection 2021. Front Oncol. 2022. PMID: 35047380 Free PMC article.
-
Retraction Note to: Mesothelial cells differentiate into fibroblast-like cells under the scirrhous gastric cancer microenvironment and promote peritoneal carcinomatosis in vitro and in vivo.Mol Cell Biochem. 2024 Jan;479(1):197-198. doi: 10.1007/s11010-023-04911-z. Mol Cell Biochem. 2024. PMID: 38038799 No abstract available.
-
PRRX1 drives tamoxifen therapy resistance through induction of epithelial-mesenchymal transition in MCF-7 breast cancer cells.Int J Clin Exp Pathol. 2018 May 1;11(5):2629-2635. eCollection 2018. Int J Clin Exp Pathol. 2018. PMID: 31938377 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

