Monocyte activation and cytokine production in Malawian children presenting with P. falciparum malaria
- PMID: 27027867
- PMCID: PMC4850749
- DOI: 10.1111/pim.12319
Monocyte activation and cytokine production in Malawian children presenting with P. falciparum malaria
Abstract
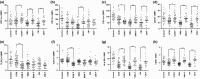
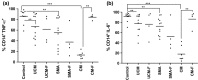
Malaria in malaria-naïve adults is associated with an inflammatory response characterized by expression of specific activation markers on innate immune cells. Here, we investigate activation and adhesion marker expression, and cytokine production in monocytes from children presenting with cerebral malaria (CM, n = 36), severe malarial anaemia (SMA, n = 42) or uncomplicated malaria (UM, n = 66), and healthy aparasitemic children (n = 52) in Blantyre, Malawi. In all malaria groups, but particularly in the two severe malaria groups, monocyte expression of CD11b, CD11c, CD18, HLA-DR and CD86, and percentages of TNF-α- and IL-6-producing monocytes were lower than in healthy controls, while expression of CD11a, TLR2 and TLR4 was lower in children with severe malaria compared with controls. These levels mostly normalized during convalescence, but percentages of cytokine-producing monocytes remained suppressed in children with SMA. In all malaria groups, especially the SMA group, a greater proportion of monocytes were loaded with haemozoin than among controls. In a P. falciparum hyperendemic area, monocytes in children with acute symptomatic malaria have reduced expression of adhesion molecules and activation markers and reduced inflammatory cytokine production. This immune suppression could be due to accumulation of haemozoin and/or previous exposure to P. falciparum.
Keywords: integrins; monocytes; toll-like receptors and cytokines.
© 2016 The Authors. Parasite Immunology Published by John Wiley & Sons Ltd.
Figures



References
-
- Langhorne J, Ndungu FM, Sponaas AM & Marsh K. Immunity to malaria: more questions than answers. Nat Immunol 2008; 9: 725–732. - PubMed
-
- Smith TG, Ayi K, Serghides L, McAllister CD & Kain KC. Innate immunity to malaria caused by Plasmodium falciparum. Clin Invest Med 2002; 25: 262–272. - PubMed
-
- Lyke KE, Burges R, Cissoko Y et al Serum levels of the proinflammatory cytokines interleukin‐1 beta (IL‐1beta), IL‐6, IL‐8, IL‐10, tumor necrosis factor alpha, and IL‐12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun 2004; 72: 5630–5637. - PMC - PubMed
-
- Riley EM. Is T‐cell priming required for initiation of pathology in malaria infections? Immunol Today 1999; 20: 228–233. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

