Allergen-Specific CD4(+) T Cells in Human Asthma
- PMID: 27027948
- PMCID: PMC5015731
- DOI: 10.1513/AnnalsATS.201507-431MG
Allergen-Specific CD4(+) T Cells in Human Asthma
Abstract
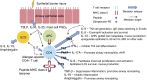
In allergic asthma, aeroallergen exposure of sensitized individuals mobilizes robust innate and adaptive airway immune responses, stimulating eosinophilic airway inflammation and the activation and infiltration of allergen-specific CD4(+) T cells into the airways. Allergen-specific CD4(+) T cells are thought to be central players in the asthmatic response as they specifically recognize the allergen and initiate and orchestrate the asthmatic inflammatory response. In this article, we briefly review the role of allergen-specific CD4(+) T cells in the pathogenesis of human allergic airway inflammation in allergic individuals, discuss the use of allergen-major histocompatibility complex class II tetramers to characterize allergen-specific CD4(+) T cells, and highlight current gaps in knowledge and directions for future research pertaining to the role of allergen-specific CD4(+) T cells in human asthma.
Keywords: CD4-positive T lymphocytes; allergens; helper T type 2 cells; humans; inflammation.
Figures
References
-
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. - PubMed
-
- Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC., Jr Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–115. - PubMed
-
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. - PubMed
-
- Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

