A novel membrane anchor for FtsZ is linked to cell wall hydrolysis in Caulobacter crescentus
- PMID: 27028265
- PMCID: PMC4935632
- DOI: 10.1111/mmi.13388
A novel membrane anchor for FtsZ is linked to cell wall hydrolysis in Caulobacter crescentus
Abstract
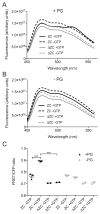
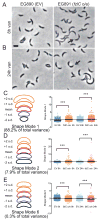
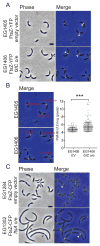
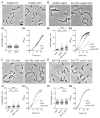
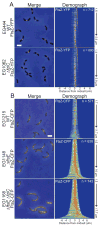
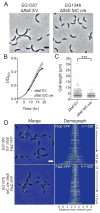
In most bacteria, the tubulin-like GTPase FtsZ forms an annulus at midcell (the Z-ring) which recruits the division machinery and regulates cell wall remodeling. Although both activities require membrane attachment of FtsZ, few membrane anchors have been characterized. FtsA is considered to be the primary membrane tether for FtsZ in bacteria, however in Caulobacter crescentus, FtsA arrives at midcell after stable Z-ring assembly and early FtsZ-directed cell wall synthesis. We hypothesized that additional proteins tether FtsZ to the membrane and demonstrate that in C. crescentus, FzlC is one such membrane anchor. FzlC associates with membranes directly in vivo and in vitro and recruits FtsZ to membranes in vitro. As for most known membrane anchors, the C-terminal peptide of FtsZ is required for its recruitment to membranes by FzlC in vitro and midcell recruitment of FzlC in cells. In vivo, overproduction of FzlC causes cytokinesis defects whereas deletion of fzlC causes synthetic defects with dipM, ftsE and amiC mutants, implicating FzlC in cell wall hydrolysis. Our characterization of FzlC as a novel membrane anchor for FtsZ expands our understanding of FtsZ regulators and establishes a role for membrane-anchored FtsZ in the regulation of cell wall hydrolysis.
© 2016 John Wiley & Sons Ltd.
Figures



Similar articles
-
GTPase activity regulates FtsZ ring positioning in Caulobacter crescentus.Mol Biol Cell. 2024 Jul 1;35(7):ar97. doi: 10.1091/mbc.E23-09-0365. Epub 2024 May 17. Mol Biol Cell. 2024. PMID: 38758654 Free PMC article.
-
FtsA Regulates Z-Ring Morphology and Cell Wall Metabolism in an FtsZ C-Terminal Linker-Dependent Manner in Caulobacter crescentus.J Bacteriol. 2020 Mar 11;202(7):e00693-19. doi: 10.1128/JB.00693-19. Print 2020 Mar 11. J Bacteriol. 2020. PMID: 31932314 Free PMC article.
-
The intrinsically disordered C-terminal linker of FtsZ regulates protofilament dynamics and superstructure in vitro.J Biol Chem. 2017 Dec 15;292(50):20509-20527. doi: 10.1074/jbc.M117.809939. Epub 2017 Oct 31. J Biol Chem. 2017. PMID: 29089389 Free PMC article.
-
A new factor stimulating peptidoglycan hydrolysis to separate daughter cells in Caulobacter crescentus.Mol Microbiol. 2010 Jul 1;77(1):11-4. doi: 10.1111/j.1365-2958.2010.07225.x. Epub 2010 May 24. Mol Microbiol. 2010. PMID: 20497501 Review.
-
Form and function of the bacterial cytokinetic ring.Curr Opin Cell Biol. 2014 Feb;26:19-27. doi: 10.1016/j.ceb.2013.08.006. Epub 2013 Sep 25. Curr Opin Cell Biol. 2014. PMID: 24529242 Review.
Cited by
-
GTPase activity regulates FtsZ ring positioning in Caulobacter crescentus.Mol Biol Cell. 2024 Jul 1;35(7):ar97. doi: 10.1091/mbc.E23-09-0365. Epub 2024 May 17. Mol Biol Cell. 2024. PMID: 38758654 Free PMC article.
-
Assembly and activation of the Escherichia coli divisome.Mol Microbiol. 2017 Jul;105(2):177-187. doi: 10.1111/mmi.13696. Epub 2017 May 25. Mol Microbiol. 2017. PMID: 28419603 Free PMC article. Review.
-
Anchors: A way for FtsZ filaments to stay membrane bound.Mol Microbiol. 2023 Oct;120(4):525-538. doi: 10.1111/mmi.15067. Epub 2023 Apr 28. Mol Microbiol. 2023. PMID: 37503768 Free PMC article. Review.
-
ZipN is an essential FtsZ membrane tether and contributes to the septal localization of SepJ in the filamentous cyanobacterium Anabaena.Sci Rep. 2019 Feb 26;9(1):2744. doi: 10.1038/s41598-019-39336-6. Sci Rep. 2019. PMID: 30808920 Free PMC article.
-
Non-essentiality of canonical cell division genes in the planctomycete Planctopirus limnophila.Sci Rep. 2020 Jan 9;10(1):66. doi: 10.1038/s41598-019-56978-8. Sci Rep. 2020. PMID: 31919386 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

