Dual Bronchodilator Therapy with Umeclidinium/Vilanterol Versus Tiotropium plus Indacaterol in Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial
- PMID: 27028749
- PMCID: PMC4875926
- DOI: 10.1007/s40268-016-0131-2
Dual Bronchodilator Therapy with Umeclidinium/Vilanterol Versus Tiotropium plus Indacaterol in Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial
Abstract
Introduction: The fixed-dose, long-acting bronchodilator combination of umeclidinium/vilanterol (UMEC/VI) has not previously been compared with a combination of a long-acting muscarinic antagonist and long-acting β2-agonist in patients with chronic obstructive pulmonary disease (COPD).
Methods: This 12-week, randomized, blinded, triple-dummy, parallel-group, non-inferiority study compared once-daily UMEC/VI 62.5/25 mcg with once-daily tiotropium (TIO) 18 mcg + indacaterol (IND) 150 mcg in patients with moderate-to-very-severe COPD. The primary endpoint was the trough forced expiratory volume in 1 s (FEV1) on day 85 (predefined non-inferiority margin -50 mL), and the secondary endpoint was the 0- to 6-h weighted mean (WM) FEV1 on day 84. Other efficacy endpoints [including rescue medication use, the Transition Dyspnea Index (TDI) focal score, and the St. George's Respiratory Questionnaire (SGRQ) score] and safety endpoints [adverse events (AEs), vital signs, and COPD exacerbations] were also assessed.
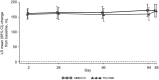
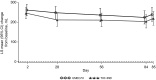
Results: Trough FEV1 improvements were comparable between treatment groups [least squares (LS) mean changes from baseline to day 85: UMEC/VI 172 mL; TIO + IND 171 mL; treatment difference 1 mL; 95 % confidence interval (CI) -29 to 30 mL], demonstrating non-inferiority between UMEC/VI and TIO + IND. The treatments produced similar improvements in the trough FEV1 at other study visits and the 0- to 6-h WM FEV1 (LS mean changes at day 84: UMEC/VI 235 mL; TIO + IND 258 mL; treatment difference -23 mL; 95 % CI -54 to 8 mL). The results for patient-reported measures (rescue medication use, TDI focal score, and SGRQ score) were comparable; both treatments produced clinically meaningful improvements in TDI and SGRQ scores. The incidence of AEs and COPD exacerbations, and changes in vital signs were similar for the two treatments.
Conclusion: UMEC/VI and TIO + IND, given once daily, provided similar improvements in lung function and patient-reported outcomes over 12 weeks in patients with COPD, with comparable tolerability and safety profiles.
Trial numbers: ClinicalTrials.gov study ID NCT02257385; GSK study no. 116961.
Figures

References
-
- Global Initiative for Chronic Obstructive Lung Disease [GOLD]. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, 2015. Available at http://www.goldcopd.com. Accessed on 03 Dec 2015. - PubMed
-
- GSK. Anoro™ ELLIPTA™ prescribing information, 2014. Available at https://www.gsksource.com/gskprm/htdocs/documents/ANORO-ELLIPTA-PI-MG.PDF. Accessed on 03 Dec 2015.
-
- GSK. Anoro™ ELLIPTA™ summary of product characteristics, 2014. Available at http://www.medicines.org.uk/emc/medicine/28949#INDICATIONS. Accessed on 03 Dec 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

