What can imaging tell us about cognitive impairment and dementia?
- PMID: 27029053
- PMCID: PMC4807333
- DOI: 10.4329/wjr.v8.i3.240
What can imaging tell us about cognitive impairment and dementia?
Abstract
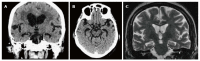
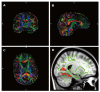
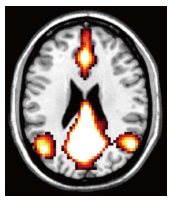
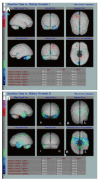
Dementia is a contemporary global health issue with far reaching consequences, not only for affected individuals and their families, but for national and global socio-economic conditions. The hallmark feature of dementia is that of irreversible cognitive decline, usually affecting memory, and impaired activities of daily living. Advances in healthcare worldwide have facilitated longer life spans, increasing the risks of developing cognitive decline and dementia in late life. Dementia remains a clinical diagnosis. The role of structural and molecular neuroimaging in patients with dementia is primarily supportive role rather than diagnostic, American and European guidelines recommending imaging to exclude treatable causes of dementia, such as tumor, hydrocephalus or intracranial haemorrhage, but also to distinguish between different dementia subtypes, the commonest of which is Alzheimer's disease. However, this depends on the availability of these imaging techniques at individual centres. Advanced magnetic resonance imaging (MRI) techniques, such as functional connectivity MRI, diffusion tensor imaging and magnetic resonance spectroscopy, and molecular imaging techniques, such as 18F fluoro-deoxy glucose positron emission tomography (PET), amyloid PET, tau PET, are currently within the realm of dementia research but are available for clinical use. Increasingly the research focus is on earlier identification of at risk preclinical individuals, for example due to family history. Intervention at the preclinical stages before irreversible brain damage occurs is currently the best hope of reducing the impact of dementia.
Keywords: Alzheimer’s disease; Dementia; Frontotemporal dementia; Lewy body dementia; Magnetic resonance imaging; Molecular imaging; Vascular dementia.
Figures
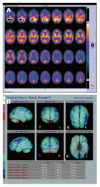
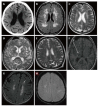
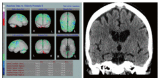
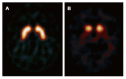

References
-
- Available from: https://www.alz.co.uk/research/WorldAlzheimerReport2014.pdf.
-
- Spijker J, MacInnes J. Population ageing: the timebomb that isn’t? BMJ. 2013;347:f6598. - PubMed
-
- Haub C. World population aging: clocks illustrate growth in population under age5 and over age 65. Population Bulletin. Available from: http: //www.prb.org/Articles/2011/agingpopulationclocks.aspx.
-
- Qiu C, Xu W, Fratiglioni L. Vascular and psychosocial factors in Alzheimer’s disease: epidemiological evidence toward intervention. J Alzheimers Dis. 2010;20:689–697. - PubMed
-
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

