Sitagliptin use and thyroid cancer risk in patients with type 2 diabetes
- PMID: 27029076
- PMCID: PMC5029749
- DOI: 10.18632/oncotarget.8399
Sitagliptin use and thyroid cancer risk in patients with type 2 diabetes
Abstract
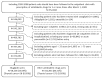
Whether sitagliptin may increase thyroid cancer risk has not been investigated in the Asian populations. This study evaluated the association in Taiwanese patients with newly diagnosed type 2 diabetes from 1999 to 2008 by using the reimbursement database of the National Health Insurance. They should have been followed for at least 6 months after March 1, 2009, the date when sitagliptin was approved for reimbursement. Patients newly treated with sitagliptin (n=58238, "ever users of sitagliptin") or other antidiabetic drugs (n =312853, "never users of sitagliptin") were followed until December 31, 2011. The treatment effect (for ever versus never users, and for tertiles of cumulative duration of therapy) was estimated by Cox regression incorporated with the inverse probability of treatment weighting using propensity score. Results showed that the respective number of incident thyroid cancer in ever users and never users was 28 and 172, with respective incidence of 29.34 and 22.13 per 100,000 person-years. The overall hazard ratio (95% confidence interval) of 1.516 (1.011-2.271) suggested a significantly higher risk associated with sitagliptin use. In tertile analyses, the hazard ratio for the first ( < 6.53 months), second (6.53-14.00 months) and third ( > 14 months) tertile of cumulative duration was 1.995 (1.015-3.919), 2.516 (1.451-4.364) and 0.595 (0.244-1.449), respectively. Analyses after excluding patients with benign thyroid disease and in a subsample matched on baseline characteristics supported the findings in the original sample. In conclusion, sitagliptin use is associated with an increased risk of thyroid cancer, especially during the first year of its treatment.
Keywords: Taiwan; diabetes mellitus; sitagliptin; thyroid cancer.
Conflict of interest statement
CONFLICTS OF INTERESTS There is no conflict of interest.
Figures
Similar articles
-
Sitagliptin may reduce prostate cancer risk in male patients with type 2 diabetes.Oncotarget. 2017 Mar 21;8(12):19057-19064. doi: 10.18632/oncotarget.12137. Oncotarget. 2017. PMID: 27661113 Free PMC article.
-
Sitagliptin and heart failure hospitalization in patients with type 2 diabetes.Oncotarget. 2016 Sep 20;7(38):62687-62696. doi: 10.18632/oncotarget.10507. Oncotarget. 2016. PMID: 27409676 Free PMC article.
-
Sitagliptin May Reduce Breast Cancer Risk in Women With Type 2 Diabetes.Clin Breast Cancer. 2017 Jun;17(3):211-218. doi: 10.1016/j.clbc.2016.11.002. Epub 2016 Nov 23. Clin Breast Cancer. 2017. PMID: 27986440
-
Sitagliptin for Type 2 diabetes: a 2015 update.Expert Rev Cardiovasc Ther. 2015 Jun;13(6):597-610. doi: 10.1586/14779072.2015.1046840. Expert Rev Cardiovasc Ther. 2015. PMID: 26000559 Review.
-
Dementia Risk in Type 2 Diabetes Patients: Acarbose Use and Its Joint Effects with Metformin and Pioglitazone.Aging Dis. 2020 May 9;11(3):658-667. doi: 10.14336/AD.2019.0621. eCollection 2020 May. Aging Dis. 2020. PMID: 32489710 Free PMC article. Review.
Cited by
-
Real-world evidence on the association of novel antidiabetic medication use with cancer risk and protective effects: a systematic review and network meta-analysis.Ther Adv Drug Saf. 2025 Apr 21;16:20420986251335214. doi: 10.1177/20420986251335214. eCollection 2025. Ther Adv Drug Saf. 2025. PMID: 40290515 Free PMC article.
-
Risk of cancer in long-term levothyroxine users: Retrospective population-based study.Cancer Sci. 2021 Jun;112(6):2533-2541. doi: 10.1111/cas.14908. Epub 2021 May 2. Cancer Sci. 2021. PMID: 33793038 Free PMC article.
-
Drug repositioning in thyroid cancer treatment: the intriguing case of anti-diabetic drugs.Front Pharmacol. 2023 Dec 11;14:1303844. doi: 10.3389/fphar.2023.1303844. eCollection 2023. Front Pharmacol. 2023. PMID: 38146457 Free PMC article. Review.
-
Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders.Endocr Rev. 2019 Jun 1;40(3):789-824. doi: 10.1210/er.2018-00163. Endocr Rev. 2019. PMID: 30649221 Free PMC article. Review.
-
The role of insulin and incretin-based drugs in biliary tract cancer: epidemiological and experimental evidence.Discov Oncol. 2022 Aug 7;13(1):70. doi: 10.1007/s12672-022-00536-8. Discov Oncol. 2022. PMID: 35933633 Free PMC article. Review.
References
-
- Tseng CH, Lee KY, Tseng FH. An updated review on cancer risk associated with incretin mimetics and enhancers. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33:67–124. - PubMed
-
- Vangoitsenhoven R, Mathieu C, Van der Schueren B. GLP1 and cancer: friend or foe? Endocr Relat Cancer. 2012;19:F77–88. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical