Immuno-physiological adaptations confer wax moth Galleria mellonella resistance to Bacillus thuringiensis
- PMID: 27029421
- PMCID: PMC5160394
- DOI: 10.1080/21505594.2016.1164367
Immuno-physiological adaptations confer wax moth Galleria mellonella resistance to Bacillus thuringiensis
Abstract
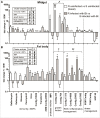
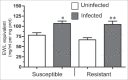
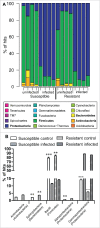
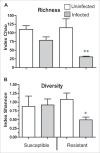
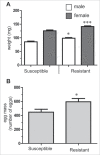
Microevolutionary mechanisms of resistance to a bacterial pathogen were explored in a population of the Greater wax moth, Galleria mellonella, selected for an 8.8-fold increased resistance against the entomopathogenic bacterium Bacillus thuringiensis (Bt) compared with a non-selected (suspectible) line. Defense strategies of the resistant and susceptible insect lines were compared to uncover mechanisms underpinning resistance, and the possible cost of those survival strategies. In the uninfected state, resistant insects exhibited enhanced basal expression of genes related to regeneration and amelioration of Bt toxin activity in the midgut. In addition, these insects also exhibited elevated activity of genes linked to inflammation/stress management and immune defense in the fat body. Following oral infection with Bt, the expression of these genes was further elevated in the fat body and midgut of both lines and to a greater extent some of them in resistant line than the susceptible line. This gene expression analysis reveals a pattern of resistance mechanisms targeted to sites damaged by Bt with the insect placing greater emphasis on tissue repair as revealed by elevated expression of these genes in both the fat body and midgut epithelium. Unlike the susceptible insects, Bt infection significantly reduced the diversity and richness (abundance) of the gut microbiota in the resistant insects. These observations suggest that the resistant line not only has a more intact midgut but is secreting antimicrobial factors into the gut lumen which not only mitigate Bt activity but also affects the viability of other gut bacteria. Remarkably the resistant line employs multifactorial adaptations for resistance to Bt without any detected negative trade off since the insects exhibited higher fecundity.
Keywords: Bt; experimental evolution; immune response; insect; microevolution; resistance.
Figures

Comment in
-
More wrinkles to Bt susceptibility.Virulence. 2016 Nov 16;7(8):853-855. doi: 10.1080/21505594.2016.1244596. Epub 2016 Oct 7. Virulence. 2016. PMID: 27715450 Free PMC article. No abstract available.
References
-
- Janmaat AF, Bergmann L, Ericsson J. Effect of low levels of Bacillus thuringiensis exposure on the growth, food consumption and digestion efficiencies of Trichoplusia ni resistant and susceptible to Bt. J Invertebrate Pathol 2014; 119:32-9; PMID:24727193; http://dx.doi.org/ 10.1016/j.jip.2014.04.001 - DOI - PubMed
-
- Paris M, David JP, Despres L. Fitness costs of resistance to Bti toxins in the dengue vector Aedes aegypti. Ecotoxicology 2011; 20:1184-94; PMID:21461926; http://dx.doi.org/ 10.1007/s10646-011-0663-8 - DOI - PubMed
-
- Raymond B, Johnston PR, Nielsen-LeRoux C, Lereclus D, Crickmore N. Bacillus thuringiensis: an impotent pathogen? Trends Microbiol 2010; 18:189-94; PMID:20338765; http://dx.doi.org/ 10.1016/j.tim.2010.02.006 - DOI - PubMed
-
- Nielsen-LeRoux C, Gaudriault S, Ramarao N, Lereclus D, Givaudan A. How the insect pathogen bacteria Bacillus thuringiensis and Xenorhabdus/Photorhabdus occupy their hosts. Curr Opin Microbiol 2012; 15:220-31; PMID:22633889; http://dx.doi.org/ 10.1016/j.mib.2012.04.006 - DOI - PubMed
-
- Slamti L, Perchat S, Huillet E, Lereclus D. Quorum Sensing in Bacillus thuringiensis Is Required for Completion of a Full Infectious Cycle in the Insect. Toxins 2014; 6:2239-55; PMID:25089349; http://dx.doi.org/ 10.3390/toxins6082239 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
