Andrographolide inhibits prostate cancer by targeting cell cycle regulators, CXCR3 and CXCR7 chemokine receptors
- PMID: 27029529
- PMCID: PMC4845948
- DOI: 10.1080/15384101.2016.1148836
Andrographolide inhibits prostate cancer by targeting cell cycle regulators, CXCR3 and CXCR7 chemokine receptors
Abstract
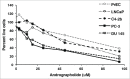
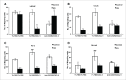
Despite state of the art cancer diagnostics and therapies offered in clinic, prostate cancer (PCa) remains the second leading cause of cancer-related deaths. Hence, more robust therapeutic/preventive regimes are required to combat this lethal disease. In the current study, we have tested the efficacy of Andrographolide (AG), a bioactive diterpenoid isolated from Andrographis paniculata, against PCa. This natural agent selectively affects PCa cell viability in a dose and time-dependent manner, without affecting primary prostate epithelial cells. Furthermore, AG showed differential effect on cell cycle phases in LNCaP, C4-2b and PC3 cells compared to retinoblastoma protein (RB(-/-)) and CDKN2A lacking DU-145 cells. G2/M transition was blocked in LNCaP, C4-2b and PC3 after AG treatment whereas DU-145 cells failed to transit G1/S phase. This difference was primarily due to differential activation of cell cycle regulators in these cell lines. Levels of cyclin A2 after AG treatment increased in all PCa cells line. Cyclin B1 levels increased in LNCaP and PC3, decreased in C4-2b and showed no difference in DU-145 cells after AG treatment. AG decreased cyclin E2 levels only in PC3 and DU-145 cells. It also altered Rb, H3, Wee1 and CDC2 phosphorylation in PCa cells. Intriguingly, AG reduced cell viability and the ability of PCa cells to migrate via modulating CXCL11 and CXCR3 and CXCR7 expression. The significant impact of AG on cellular and molecular processes involved in PCa progression suggests its potential use as a therapeutic and/or preventive agent for PCa.
Keywords: Andrographolide; CXCL11; CXCR3; CXCR7; Cell cycle; Cyclins; chemokine; chemokine receptor and prostate cancer.
Figures




Similar articles
-
HOXB13 contributes to G1/S and G2/M checkpoint controls in prostate.Mol Cell Endocrinol. 2014 Mar 5;383(1-2):38-47. doi: 10.1016/j.mce.2013.12.003. Epub 2013 Dec 8. Mol Cell Endocrinol. 2014. PMID: 24325868
-
CXCL11 promotes tumor progression by the biased use of the chemokine receptors CXCR3 and CXCR7.Cytokine. 2020 Jan;125:154809. doi: 10.1016/j.cyto.2019.154809. Epub 2019 Aug 19. Cytokine. 2020. PMID: 31437604
-
How can food extracts consumed in the Mediterranean and East Asia suppress prostate cancer proliferation?Br J Nutr. 2012 Aug;108(3):424-30. doi: 10.1017/S0007114511005770. Epub 2011 Nov 9. Br J Nutr. 2012. PMID: 22067725
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
-
Andrographolide, a diterpene lactone from Andrographis paniculata and its therapeutic promises in cancer.Cancer Lett. 2018 Apr 28;420:129-145. doi: 10.1016/j.canlet.2018.01.074. Epub 2018 Feb 14. Cancer Lett. 2018. PMID: 29408515
Cited by
-
NF-κB and Its Role in Checkpoint Control.Int J Mol Sci. 2020 May 31;21(11):3949. doi: 10.3390/ijms21113949. Int J Mol Sci. 2020. PMID: 32486375 Free PMC article. Review.
-
Andrographolide sensitizes prostate cancer cells to TRAIL-induced apoptosis.Asian J Androl. 2018 Mar-Apr;20(2):200-204. doi: 10.4103/aja.aja_30_17. Asian J Androl. 2018. PMID: 28869219 Free PMC article.
-
Transcriptome Network Analysis Identifies CXCL13-CXCR5 Signaling Modules in the Prostate Tumor Immune Microenvironment.Sci Rep. 2019 Oct 18;9(1):14963. doi: 10.1038/s41598-019-46491-3. Sci Rep. 2019. PMID: 31628349 Free PMC article.
-
Advances in Molecular Regulation of Prostate Cancer Cells by Top Natural Products of Malaysia.Curr Issues Mol Biol. 2023 Feb 9;45(2):1536-1567. doi: 10.3390/cimb45020099. Curr Issues Mol Biol. 2023. PMID: 36826044 Free PMC article. Review.
-
Natural and Synthetic Lactones Possessing Antitumor Activities.Int J Mol Sci. 2021 Jan 21;22(3):1052. doi: 10.3390/ijms22031052. Int J Mol Sci. 2021. PMID: 33494352 Free PMC article. Review.
References
-
- Saklani A, Kutty SK. Plant-derived compounds in clinical trials. Drug Discov Today 2008; 13:161-71; PMID:18275914; http://dx.doi.org/10.1016/j.drudis.2007.10.010 - DOI - PubMed
-
- Gao S, Hu M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini Rev Med Chem 2010; 10:550-67; PMID:20370701; http://dx.doi.org/10.2174/138955710791384081 - DOI - PMC - PubMed
-
- Panossian A, Hovhannisyan A, Mamikonyan G, Abrahamian H, Hambardzumyan E, Gabrielian E, Goukasova G, Wikman G, Wagner H. Pharmacokinetic and oral bioavailability of andrographolide from andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine: International Journal of phytotherapy and phytopharmacology 2000; 7:351-64; PMID:11081986; http://dx.doi.org/10.1016/S0944-7113(00)80054-9 - DOI - PubMed
-
- Chen HW, Huang CS, Li CC, Lin AH, Huang YJ, Wang TS, Yao HT, Lii CK. Bioavailability of andrographolide and protection against carbon tetrachloride-induced oxidative damage in rats. Toxicol Appl Pharm 2014; 280:1-9; PMID:NOT_FOUND; http://dx.doi.org/10.1016/j.taap.2014.07.024 - DOI - PubMed
-
- Rajagopal S, Kumar RA, Deevi DS, Satyanarayana C, Rajagopalan R. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J Exp Ther Oncol 2003; 3:147-58; PMID:14641821; http://dx.doi.org/10.1046/j.1359-4117.2003.01090.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
