Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence
- PMID: 27029708
- PMCID: PMC4922316
- DOI: 10.1093/annonc/mdw150
Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence
Abstract
Background: AE37 is the Ii-Key hybrid of the MHC class II peptide, AE36 (HER2 aa:776-790). Phase I studies showed AE37 administered with granulocyte macrophage colony-stimulating factor (GM-CSF) to be safe and highly immunogenic. A prospective, randomized, multicenter phase II adjuvant trial was conducted to evaluate the vaccine's efficacy.
Methods: Clinically disease-free node-positive and high-risk node-negative breast cancer patients with tumors expressing any degree of HER2 [immunohistochemistry (IHC) 1-3+] were enrolled. Patients were randomized to AE37 + GM-CSF versus GM-CSF alone. Toxicity was monitored. Clinical recurrences were documented and disease-free survival (DFS) analyzed.
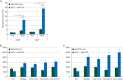
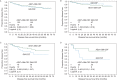
Results: The trial enrolled 298 patients; 153 received AE37 + GM-CSF and 145 received GM-CSF alone. The groups were well matched for clinicopathologic characteristics. Toxicities have been minimal. At the time of the primary analysis, the recurrence rate in the vaccinated group was 12.4% versus 13.8% in the control group [relative risk reduction 12%, HR 0.885, 95% confidence interval (CI) 0.472-1.659, P = 0.70]. The Kaplan-Meier estimated 5-year DFS rate was 80.8% in vaccinated versus 79.5% in control patients. In planned subset analyses of patients with IHC 1+/2+ HER2-expressing tumors, 5-year DFS was 77.2% in vaccinated patients (n = 76) versus 65.7% in control patients (n = 78) (P = 0.21). In patients with triple-negative breast cancer (HER2 IHC 1+/2+ and hormone receptor negative) DFS was 77.7% in vaccinated patients (n = 25) versus 49.0% in control patients (n = 25) (P = 0.12).
Conclusion: The overall intention-to-treat analysis demonstrates no benefit to vaccination. However, the results confirm that the vaccine is safe and suggest that vaccination may have clinical benefit in patients with low HER2-expressing tumors, specifically TNBC. Further evaluation in a randomized trial enrolling TNBC patients is warranted.
Keywords: HER2; breast cancer; immunotherapy; triple-negative breast cancer; vaccine.
© The Author 2016. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Peoples GE, Holmes JP, Hueman MT et al. . Combined Clinical Trial Results of a HER2/neu (E75) Vaccine for the prevention of recurrence in high-risk breast cancer patients: U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res 2008; 14: 797–803. - PubMed
-
- Knutson KL, Disis ML. Augmenting T helper cell immunity in cancer. Curr Drug Targets Immune Endocr Metabol Disord 2005; 5: 365–371. - PubMed
-
- Disis ML, Grabstein KH, Sleath PR, Cheever MA. Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res 1999; 5: 1289–1297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

