Decarboxylative Cross-Electrophile Coupling of N-Hydroxyphthalimide Esters with Aryl Iodides
- PMID: 27029833
- PMCID: PMC4841236
- DOI: 10.1021/jacs.6b01533
Decarboxylative Cross-Electrophile Coupling of N-Hydroxyphthalimide Esters with Aryl Iodides
Abstract
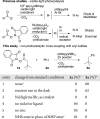
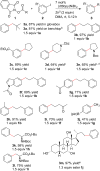
A new method for the decarboxylative coupling of alkyl N-hydroxyphthalimide esters (NHP esters) with aryl iodides is presented. In contrast to previous studies that form alkyl radicals from carboxylic acid derivatives, no photocatalyst, light, or arylmetal reagent is needed, only nickel and a reducing agent (Zn). Methyl, primary, and secondary alkyl groups can all be coupled in good yield (77% ave yield). One coupling with an acid chloride is also presented. Stoichiometric reactions of (dtbbpy)Ni(2-tolyl)I with an NHP ester show for the first time that arylnickel(II) complexes can directly react with NHP esters to form alkylated arenes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Frisch A. C.; Beller M. Angew. Chem., Int. Ed. 2005, 44, 674–688. 10.1002/anie.200461432. - DOI - PubMed
- Rudolph A.; Lautens M. Angew. Chem., Int. Ed. 2009, 48, 2656–2670. 10.1002/anie.200803611. - DOI - PubMed
- Jana R.; Pathak T. P.; Sigman M. S. Chem. Rev. 2011, 111, 1417–1492. 10.1021/cr100327p. - DOI - PMC - PubMed
- Manolikakes G.3.08 Coupling Reactions Between sp3 and sp2 Carbon Centers A2. In Comprehensive Organic Synthesis II, 2nd ed.; Knochel P., Ed.; Elsevier: Amsterdam, 2014; pp 392–464.
-
-
While 318,496 Ar-Br and 1,000,000 Ar-Cl are listed as commercially available in the eMolecules database (accessed via REAXYS 6/22/2015), only 9,282 alkyl-Br and 24,746 alkyl-Cl are listed. In contrast, there are 83,315 alkanoic acids commercially available.
-
-
- Dieter R. K. Tetrahedron 1999, 55, 4177–4236. 10.1016/S0040-4020(99)00184-2. - DOI
-
-
The decarbonylative coupling of alkanoic acid derivatives has more precedent; see the following two examples and references therein:
- O’Brien E. M.; Bercot E. A.; Rovis T. J. Am. Chem. Soc. 2003, 125, 10498–10499. 10.1021/ja036290b. - DOI - PubMed
- Muto K.; Yamaguchi J.; Musaev D. G.; Itami K. Nat. Commun. 2015, 6, 7508.10.1038/ncomms8508. - DOI - PMC - PubMed
-
-
- Zuo Z.; Ahneman D.; Chu L.; Terrett J.; Doyle A. G.; MacMillan D. W. C. Science 2014, 345, 437–440. 10.1126/science.1255525. - DOI - PMC - PubMed
- Noble A.; McCarver S. J.; MacMillan D. W. C. J. Am. Chem. Soc. 2015, 137, 624–627. 10.1021/ja511913h. - DOI - PMC - PubMed
- Luo J.; Zhang J. ACS Catal. 2016, 6, 873–877. 10.1021/acscatal.5b02204. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

