CAMERA2 - combination antibiotic therapy for methicillin-resistant Staphylococcus aureus infection: study protocol for a randomised controlled trial
- PMID: 27029920
- PMCID: PMC4815121
- DOI: 10.1186/s13063-016-1295-3
CAMERA2 - combination antibiotic therapy for methicillin-resistant Staphylococcus aureus infection: study protocol for a randomised controlled trial
Abstract
Background: Methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia is a serious infection resulting in 20-50 % 90-day mortality. The limitations of vancomycin, the current standard therapy for MRSA, make treatment difficult. The only other approved drug for treatment of MRSA bacteraemia, daptomycin, has not been shown to be superior to vancomycin. Surprisingly, there has been consistent in-vitro and in-vivo laboratory data demonstrating synergy between vancomycin or daptomycin and an anti-staphylococcal β-lactam antibiotic. There is also growing clinical data to support such combinations, including a recent pilot randomised controlled trial (RCT) that demonstrated a trend towards a reduction in the duration of bacteraemia in patients treated with vancomycin plus flucloxacillin compared to vancomycin alone. Our aim is to determine whether the addition of an anti-staphylococcal penicillin to standard therapy results in improved clinical outcomes in MRSA bacteraemia.
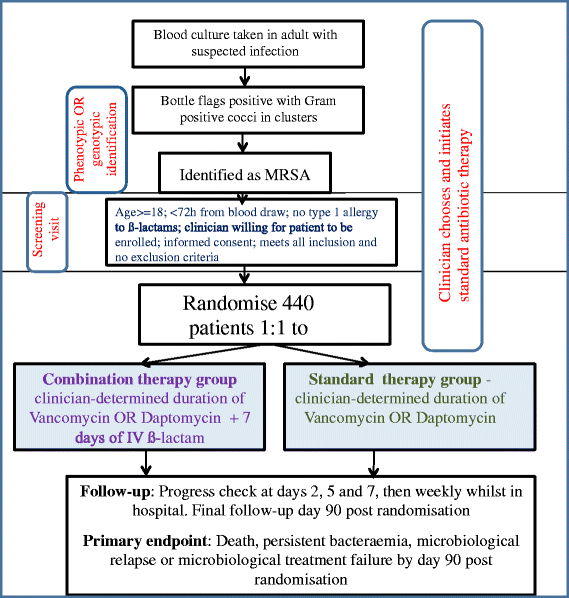
Methods/design: We will perform an open-label, parallel-group, randomised (1:1) controlled trial at 29 sites in Australia, New Zealand, Singapore, and Israel. Adults (aged 18 years or older) with MRSA grown from at least one blood culture and able to be randomised within 72 hours of the index blood culture collection will be eligible for inclusion. Participants will be randomised to vancomycin or daptomycin (standard therapy) given intravenously or to standard therapy plus 7 days of an anti-staphylococcal β-lactam (flucloxacillin, cloxacillin, or cefazolin). The primary endpoint will be a composite outcome at 90 days of (1) all-cause mortality, (2) persistent bacteraemia at day 5 or beyond, (3) microbiological relapse, or (4) microbiological treatment failure. The recruitment target of 440 patients is based on an expected failure rate for the primary outcome of 30 % in the control arm and the ability to detect a clinically meaningful absolute decrease of 12.5 %, with a two-sided alpha of 0.05, a power of 80 %, and assuming 10 % of patients will not be evaluable for the primary endpoint.
Discussion: Key potential advantages of adding anti-staphylococcal β-lactams to standard therapy for MRSA bacteraemia include their safety profile, low cost, and wide availability.
Trial registration: ClinicalTrials.gov Identifier: NCT02365493 . Registered 24 February 2015.
Keywords: Cefazolin; Cloxacillin; Combination; Daptomycin; Flucloxacillin; MRSA; Methicillin-resistant; Nafcillin; Randomised controlled trial; Staphylococcus aureus; Vancomycin.
References
-
- Turnidge JD, Kotsanas D, Munckhof W, Roberts S, Bennett CM, Nimmo GR, et al. Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med J Aust. 2009;191:368–73. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical