Gene Electrotransfer: A Mechanistic Perspective
- PMID: 27029943
- PMCID: PMC5412002
- DOI: 10.2174/1566523216666160331130040
Gene Electrotransfer: A Mechanistic Perspective
Abstract
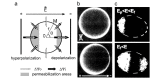
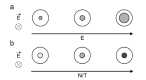
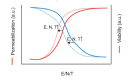
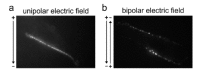
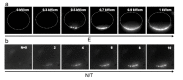
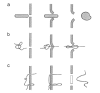
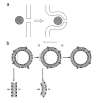
Gene electrotransfer is a powerful method of DNA delivery offering several medical applications, among the most promising of which are DNA vaccination and gene therapy for cancer treatment. Electroporation entails the application of electric fields to cells which then experience a local and transient change of membrane permeability. Although gene electrotransfer has been extensively studied in in vitro and in vivo environments, the mechanisms by which DNA enters and navigates through cells are not fully understood. Here we present a comprehensive review of the body of knowledge concerning gene electrotransfer that has been accumulated over the last three decades. For that purpose, after briefly reviewing the medical applications that gene electrotransfer can provide, we outline membrane electropermeabilization, a key process for the delivery of DNA and smaller molecules. Since gene electrotransfer is a multipart process, we proceed our review in describing step by step our current understanding, with particular emphasis on DNA internalization and intracellular trafficking. Finally, we turn our attention to in vivo testing and methodology for gene electrotransfer.
Figures


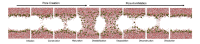


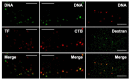
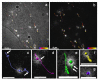
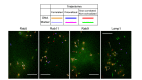
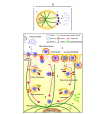
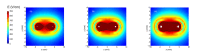
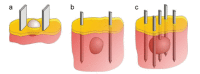
References
-
- Rogers S. Gene therapy: a potentially invaluable aid to medicine and mankind. Res. Commun. Chem. Pathol. Pharmacol. 1971;2(4):587–600. - PubMed
-
- Cian M.M., Helen O.M. Cancer Gene Therapy – Key Biological Concepts in the Design of Multifunctional Non-Viral Delivery Systems. INTECH Open Access Publisher; 2013.
-
- Greco O, Scott SD, Marples B, et al. Cancer gene therapy: 'delivery, delivery, delivery'. . Front Biosci . 2002. - PubMed
-
- Xiao P.J., Lentz T.B., Samulski R.J. Recombinant adeno-associated virus: clinical application and development as a gene-therapy vector. Ther. Deliv. 2012;3(7):835–856. - PubMed
-
- Bester A.C., Schwartz M., Schmidt M., et al. Fragile sites are preferential targets for integrations of MLV vectors in gene therapy. Gene Ther. 2006;13(13):1057–1059. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
