pH Induced Conformational Transitions in the Transforming Growth Factor β-Induced Protein (TGFβIp) Associated Corneal Dystrophy Mutants
- PMID: 27030015
- PMCID: PMC4814907
- DOI: 10.1038/srep23836
pH Induced Conformational Transitions in the Transforming Growth Factor β-Induced Protein (TGFβIp) Associated Corneal Dystrophy Mutants
Abstract
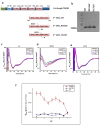
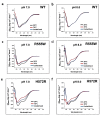
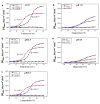
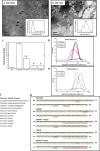
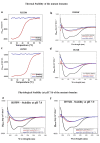
Most stromal corneal dystrophies are associated with aggregation and deposition of the mutated transforming growth factor-β induced protein (TGFβIp). The 4(th)_FAS1 domain of TGFβIp harbors ~80% of the mutations that forms amyloidogenic and non-amyloidogenic aggregates. To understand the mechanism of aggregation and the differences between the amyloidogenic and non-amyloidogenic phenotypes, we expressed the 4(th)_FAS1 domains of TGFβIp carrying the mutations R555W (non-amyloidogenic) and H572R (amyloidogenic) along with the wild-type (WT). R555W was more susceptible to acidic pH compared to H572R and displayed varying chemical stabilities with decreasing pH. Thermal denaturation studies at acidic pH showed that while WT did not undergo any conformational transition, the mutants exhibited a clear pH-dependent irreversible conversion from αβ conformation to β-sheet oligomers. The β-oligomers of both mutants were stable at physiological temperature and pH. Electron microscopy and dynamic light scattering studies showed that β-oligomers of H572R were larger compared to R555W. The β-oligomers of both mutants were cytotoxic to primary human corneal stromal fibroblast (pHCSF) cells. The β-oligomers of both mutants exhibit variations in their morphologies, sizes, thermal and chemical stabilities, aggregation patterns and cytotoxicities.
Figures



References
-
- Surguchev A. & Surguchov A. Conformational diseases: looking into the eyes. Brain Res. Bull 81, 12–24 (2010). - PubMed
-
- Lakshminarayanan R. et al. Biochemical properties and aggregation propensity of transforming growth factor-induced protein (TGFβIp) and the amyloid forming mutants. Ocul Surf. 13(1), 9–25 (2015). - PubMed
-
- Kitahama S. et al. Expression of fibrillins and other microfibril-associated proteins in human bone and osteoblast-like cells. Bone 27, 61–67 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

