Cross-species analysis of Fc engineered anti-Lewis-Y human IgG1 variants in human neonatal receptor transgenic mice reveal importance of S254 and Y436 in binding human neonatal Fc receptor
- PMID: 27030023
- PMCID: PMC4966839
- DOI: 10.1080/19420862.2016.1156285
Cross-species analysis of Fc engineered anti-Lewis-Y human IgG1 variants in human neonatal receptor transgenic mice reveal importance of S254 and Y436 in binding human neonatal Fc receptor
Abstract
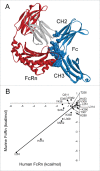
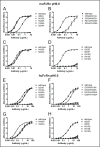
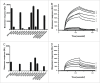
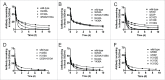
IgG has a long half-life through engagement of its Fc region with the neonatal Fc receptor (FcRn). The FcRn binding site on IgG1 has been shown to contain I253 and H310 in the CH2 domain and H435 in the CH3 domain. Altering the half-life of IgG has been pursued with the aim to prolong or reduce the half-life of therapeutic IgGs. More recent studies have shown that IgGs bind differently to mouse and human FcRn. In this study we characterize a set of hu3S193 IgG1 variants with mutations in the FcRn binding site. A double mutation in the binding site is necessary to abrogate binding to murine FcRn, whereas a single mutation in the FcRn binding site is sufficient to no longer detect binding to human FcRn and create hu3S193 IgG1 variants with a half-life similar to previously studied hu3S193 F(ab')2 (t1/2β, I253A, 12.23 h; H310A, 12.94; H435A, 12.57; F(ab')2, 12.6 h). Alanine substitutions in S254 in the CH2 domain and Y436 in the CH3 domain showed reduced binding in vitro to human FcRn and reduced elimination half-lives in huFcRn transgenic mice (t1/2β, S254A, 37.43 h; Y436A, 39.53 h; wild-type, 83.15 h). These variants had minimal effect on half-life in BALB/c nu/nu mice (t1/2β, S254A, 119.9 h; Y436A, 162.1 h; wild-type, 163.1 h). These results provide insight into the interaction of human Fc by human FcRn, and are important for antibody-based therapeutics with optimal pharmacokinetics for payload strategies used in the clinic.
Keywords: Antibody engineering; Fc receptors; molecular biology; neonatal Fc receptor; transgenic mice.
Figures


Similar articles
-
Global conformational changes in IgG-Fc upon mutation of the FcRn-binding site are not associated with altered antibody-dependent effector functions.Biochem J. 2018 Jul 5;475(13):2179-2190. doi: 10.1042/BCJ20180139. Biochem J. 2018. PMID: 29794155
-
The neonatal Fc receptor (FcRn) binds independently to both sites of the IgG homodimer with identical affinity.MAbs. 2015;7(2):331-43. doi: 10.1080/19420862.2015.1008353. MAbs. 2015. PMID: 25658443 Free PMC article.
-
Fc Engineering of Human IgG1 for Altered Binding to the Neonatal Fc Receptor Affects Fc Effector Functions.J Immunol. 2015 Jun 1;194(11):5497-508. doi: 10.4049/jimmunol.1401218. Epub 2015 Apr 22. J Immunol. 2015. PMID: 25904551 Free PMC article.
-
Neonatal Fc receptor (FcRn): a novel target for therapeutic antibodies and antibody engineering.J Drug Target. 2014 May;22(4):269-78. doi: 10.3109/1061186X.2013.875030. Epub 2014 Jan 9. J Drug Target. 2014. PMID: 24404896 Review.
-
Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity?Mol Immunol. 2013 Dec;56(4):660-74. doi: 10.1016/j.molimm.2013.05.008. Epub 2013 Aug 2. Mol Immunol. 2013. PMID: 23917469 Review.
Cited by
-
Exploring How Antibody Format Drives Clearance from the Brain.Mol Pharm. 2024 Sep 2;21(9):4416-4429. doi: 10.1021/acs.molpharmaceut.4c00354. Epub 2024 Jul 26. Mol Pharm. 2024. PMID: 39058284
-
Engineering anti-Lewis-Y hu3S193 antibodies with improved therapeutic ratio for radioimmunotherapy of epithelial cancers.EJNMMI Res. 2016 Dec;6(1):26. doi: 10.1186/s13550-016-0180-0. Epub 2016 Mar 17. EJNMMI Res. 2016. PMID: 26983636 Free PMC article.
-
Recent Achievements and Challenges in Prolonging the Serum Half-Lives of Therapeutic IgG Antibodies Through Fc Engineering.BioDrugs. 2021 Mar;35(2):147-157. doi: 10.1007/s40259-021-00471-0. Epub 2021 Feb 19. BioDrugs. 2021. PMID: 33608823 Free PMC article. Review.
-
Impact of antibody Fc engineering on translational pharmacology, and safety: insights from industry case studies.MAbs. 2025 Dec;17(1):2505092. doi: 10.1080/19420862.2025.2505092. Epub 2025 Jul 7. MAbs. 2025. PMID: 40624840 Free PMC article. Review.
-
Decoupling FcRn and tumor contributions to elevated immune checkpoint inhibitor clearance in cancer cachexia.Pharmacol Res. 2024 Jan;199:107048. doi: 10.1016/j.phrs.2023.107048. Epub 2023 Dec 23. Pharmacol Res. 2024. PMID: 38145833 Free PMC article.
References
-
- Brambell FW. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet 1966; 2:1087-93; PMID:4162525; http://dx.doi.org/10.1016/S0140-6736(66)92190-8 - DOI - PubMed
-
- Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A 1996; 93:5512-6; PMID:8643606; http://dx.doi.org/10.1073/pnas.93.11.5512 - DOI - PMC - PubMed
-
- Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7:715-25; PMID:17703228; http://dx.doi.org/10.1038/nri2155 - DOI - PubMed
-
- Rath T, Kuo TT, Baker K, Qiao SW, Kobayashi K, Yoshida M, Roopenian D, Fiebiger E, Lencer WI, Blumberg RS. The immunologic functions of the neonatal Fc receptor for IgG. J Clin Immunol 2013; 33 Suppl 1:S9-17; PMID:22948741; http://dx.doi.org/10.1007/s10875-012-9768-y - DOI - PMC - PubMed
-
- Wang Y, Tian Z, Thirumalai D, Zhang X. Neonatal Fc receptor (FcRn): a novel target for therapeutic antibodies and antibody engineering. J Drug Target 2014; 22(4):269-78; PMID:24404896; http://dx.doi.org/10.3109/1061186X.2013.875030 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
