Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness
- PMID: 27030166
- PMCID: PMC6485401
- DOI: 10.1002/14651858.CD011008.pub2
Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness
Abstract
Background: Breathlessness is a common and disabling symptom which affects many people with advanced cardiorespiratory disease and cancer. The most effective treatments are aimed at treating the underlying disease. However, this may not always be possible, and symptomatic treatment is often required in addition to maximal disease-directed therapy. Opioids are increasingly being used to treat breathlessness, although their mechanism of action is still not completely known. A few good sized, high quality trials have been conducted in this area.
Objectives: To determine the effectiveness of opioid drugs in relieving the symptom of breathlessness in people with advanced disease due to malignancy, respiratory or cardiovascular disease, or receiving palliative care for any other disease.
Search methods: We performed searches on CENTRAL, MEDLINE, EMBASE, CINAHL, and Web of Science up to 19 October 2015. We handsearched review articles, clinical trial registries, and reference lists of retrieved articles.
Selection criteria: We included randomised double-blind controlled trials that compared the use of any opioid drug against placebo or any other intervention for the relief of breathlessness. The intervention was any opioid, given by any route, in any dose.
Data collection and analysis: We imported studies identified by the search into a reference manager database. We retrieved the full-text version of relevant studies, and two review authors independently extracted data. The primary outcome measure was breathlessness and secondary outcome measures included exercise tolerance, oxygen saturations, adverse events, and mortality. We analysed all studies together and also performed subgroup analyses, by route of administration, type of opioid administered, and cause of breathlessness. Where appropriate, we performed meta-analysis. We assessed the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach and created three 'Summary of findings' tables.
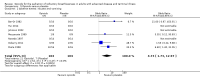
Main results: We included 26 studies with 526 participants. We assessed the studies as being at high or unclear risk of bias overall. We only included randomised controlled trials (RCTs), although the description of randomisation was incomplete in some included studies. We aimed to include double blind RCTs, but two studies were only single blinded. There was inconsistency in the reporting of outcome measures. We analysed the data using a fixed-effect model, and for some outcomes heterogeneity was high. There was a risk of imprecise results due to the low numbers of participants in the included studies. For these reasons we downgraded the quality of the evidence from high to either low or very low.For the primary outcome of breathlessness, the mean change from baseline dyspnoea score was 0.09 points better in the opioids group compared to the placebo group (ranging from a 0.36 point reduction to a 0.19 point increase) (seven RCTs, 117 participants, very low quality evidence). A lower score indicates an improvement in breathlessness. The mean post-treatment dyspnoea score was 0.28 points better in the opioid group compared to the placebo group (ranging from a 0.5 point reduction to a 0.05 point increase) (11 RCTs, 159 participants, low quality evidence).The evidence for the six-minute walk test (6MWT) was conflicting. The total distance in 6MWT was 28 metres (m) better in the opioids group compared to placebo (ranging from 113 m to 58 m) (one RCT, 11 participants, very low quality evidence). However, the change in baseline was 48 m worse in the opioids group (ranging from 36 m to 60 m) (two RCTs, 26 participants, very low quality evidence).The adverse effects reported included drowsiness, nausea and vomiting, and constipation. In those studies, participants were 4.73 times more likely to experience nausea and vomiting compared to placebo, three times more likely to experience constipation, and 2.86 times more likely to experience drowsiness (nine studies, 162 participants, very low quality evidence).Only four studies assessed quality of life, and none demonstrated any significant change.
Authors' conclusions: There is some low quality evidence that shows benefit for the use of oral or parenteral opioids to palliate breathlessness, although the number of included participants was small. We found no evidence to support the use of nebulised opioids. Further research with larger numbers of participants, using standardised protocols and with quality of life measures included, is needed.
Conflict of interest statement
HB has no relevant conflicts of interest to declare. JM has no relevant conflicts of interest to declare. NS has no relevant conflicts of interest to declare. RM has no relevant conflicts of interest to declare.
Figures
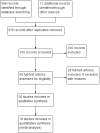
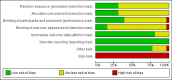
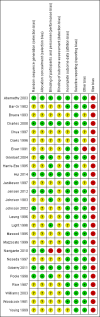


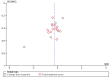
















Comment in
-
One evidence base; three stories: do opioids relieve chronic breathlessness?Thorax. 2018 Jan;73(1):88-90. doi: 10.1136/thoraxjnl-2016-209868. Epub 2017 Apr 4. Thorax. 2018. PMID: 28377491
References
References to studies included in this review
Abernethy 2003 {published data only}
-
- Currow DC, Plummer J, Frith P, Abernethy AP. Can we predict which patients with refractory dyspnea will respond to opioids?. Journal of Palliative Medicine 2007;10(5):1031‐6. - PubMed
Bar‐Or 1982 {published data only}
-
- Bar‐Or D, Marx JA, Good J. Breathlessness, alcohol and opiates. The New England Journal of Medicine 1982;306(22):1363–4. - PubMed
Bruera 1993 {published data only}
-
- Bruera E, MacEachern T, Ripamonti C, Hanson J. Subcutaneous morphine for dyspnea in cancer patients. Annals of Internal Medicine 1993;119(9):906‐7. - PubMed
Charles 2008 {published data only}
-
- Charles MA, Reymond L, Israel F. Relief of incident dyspnea in palliative cancer patients: a pilot, randomized, controlled trial comparing nebulized hydromorphone, systemic hydromorphone, and nebulized saline. Journal of Pain and Symptom Management 2008;36(1):29‐38. - PubMed
Chua 1997 {published data only}
-
- Chua T, Harrington D, Ponikowski P, Webb‐Peploe K, Poole‐Wilson P, Coats A. Effects of dihydrocodeine on chemosensitivity and exercise tolerance in patients with chronic heart failure. Journal of the American College of Cardiology 1997;29(1):147‐52. - PubMed
Davis 1996 {published data only}
-
- Davis C, Penn K, A’Hern R, Daniels J, Slevin M. Single dose randomised controlled trial of nebulised morphine in patients with cancer related breathlessness. Palliative Medicine 1996;10:64–5.
Eiser 1991 {published data only}
-
- Eiser N, Denman WT, West, C Luce P. Oral diamorphine: lack of effect on dyspnoea and exercise tolerance in the "pink puffer" syndrome. European Respiratory Journal 1991;4(8):926‐31. - PubMed
Grimbert 2004 {published data only}
-
- Grimbert D, Lubin O, Monte M, Vecellio None L, Perrier M, Carré P, et al. Dyspnea and morphine aerosols in the palliative care of lung cancer [Dyspnée et aérosols de morphine dans les soins palliatifs du cancer broncho‐pulmonaire]. Revue des Maladies Respiratoires 2004;21(6 Pt 1):1091‐7. - PubMed
Harris‐Eze 1995 {published data only}
-
- Harris‐Eze AO, Sridhar G, Clemens RE, Zintel TA, Gallagher CG, Marciniuk DD. Low‐dose nebulized morphine does not improve exercise in interstitial lung disease. American Journal of Respiratory and Critical Care Medicine 1995;152(6):1940‐5. - PubMed
Hui 2014 {published data only}
-
- Hui D, Xu A, Frisbee‐Hume S, Chisholm G, Morgado M, Reddy S, et al. Effects of prophylactic subcutaneous fentanyl on exercise‐induced breakthrough dyspnea in cancer patients: a preliminary double‐blind, randomized, controlled trial. Journal of Pain and Symptom Management 2014;47(2):209‐17. - PMC - PubMed
Jankleson 1997 {published data only}
-
- Jankelson D, Hosseini K, Mather LE, Seale JP, Young IH. Lack of effect of high doses of inhaled morphine on exercise endurance in chronic obstructive pulmonary disease. European Respiratory Journal 1997;10(10):2270–4. - PubMed
Jensen 2012 {published data only}
-
- Jensen D, Alsuhail A, Viola R, Dudgeon DJ, Webb KA, O’Donnell DE. Inhaled fentanyl citrate improves exercise endurance during high‐intensity constant work rate cycle exercise in chronic obstructive pulmonary disease. Journal of Pain and Symptom Management 2012;43(4):706‐19. - PubMed
Johnson 1983 {published data only}
Johnson 2002 {published data only}
-
- Johnson MJ, McDonagh TA, Harkness A, McKayd SE, Dargie HJ. Morphine for the relief of breathlessness in patients with chronic heart failure‐‐a pilot study. European Journal of Heart Failure 2002;4(6):753–6. - PubMed
Leung 1996 {published data only}
Light 1996 {published data only}
-
- Light RW, Stansbury DW, Webster JS. Effect of 30 mg of morphine alone or with promethazine or prochlorperazine on the exercise capacity of patients with COPD. Chest 1996;109(4):975–81. - PubMed
Masood 1995 {published data only}
Mazzocato 1999 {published data only}
-
- Mazzocato C, Buclin T, Rapin CH. The effects of morphine on dyspnea and ventilatory function in elderly patients with advanced cancer: A randomized double‐blind controlled trial. Annals of Oncology 1999;10(12):1511‐4. - PubMed
Navigante 2010 {published data only}
-
- Cerchietti LCA, Navigante, AH. Midazolam as adjunct therapy to morphine to relieve dyspnea? Authors' reply. Journal of Pain and Symptom Management 2007;33(3):234‐6. - PubMed
-
- Navigante AH, Castro MA, Cerchietti LC. Morphine versus midazolam as upfront therapy to control dyspnea perception in cancer patients while its underlying cause is sought or treated. Journal of Pain and Symptom Management 2010;39(5):820‐30. - PubMed
Noseda 1997 {published data only}
-
- Noseda A, Carpiaux JP, Markstein C, Meyvaert A, Maertelaer V. Disabling dyspnoea in patients with advanced disease: lack of effect of nebulized morphine. European Respiratory Journal 1997;10(5):1079–83. - PubMed
Oxberry 2011 {published data only}
-
- Oxberry SG, Bland JM, Clark AL, Cleland JG, Johnson MJ. Minimally clinically important difference in chronic breathlessness: every little helps. American Heart Journal 2012;164(2):229‐35. - PubMed
-
- Oxberry SG, Torgerson DJ, Bland JM, Clark AL, Cleland JG, Johnson MJ. Short‐term opioids for breathlessness in stable chronic heart failure: a randomized controlled trial. European Journal of Heart Failure 2011;13(9):1006–12. - PubMed
Poole 1998 {published data only}
-
- Poole PJ, Veale AG, Black PN. The effect of sustained‐release morphine on breathlessness and quality of life in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157(6):1877–80. - PubMed
Rice 1987 {published data only}
-
- Rice KL, Kronenberg RS, Hedemark LL, Niewoehner DE. Effects of chronic administration of codeine and promethazine on breathlessness and exercise tolerance in patients with chronic airflow obstruction. British Journal of Diseases of the Chest 1987;81(3):287–92. - PubMed
Williams 2003 {published data only}
Woodcock 1981 {published data only}
-
- Woodcock AA, Gross ER, Gellert A, Shah S, Johnson M, Geddes DM. Effects of dihydrocodeine, alcohol, and caffeine on breathlessness and exercise tolerance in patients with chronic obstructive lung disease and normal blood gases. The New England Journal of Medicine 1981;305(27):1611–6. - PubMed
References to studies excluded from this review
Allard 1999 {published data only}
-
- Allard P, Lamontagne C, Bernard P, Tremblay CJ. How effective are supplementary doses of opioids for dyspnea in terminally ill cancer patients? A randomized continuous sequential clinical trial. Journal of Pain and Symptom Management 1999;17(4):256–65. - PubMed
Beauford 1993 {published data only}
-
- Beauford W, Saylor TT, Stansbury DW, Avalos K, Light RW. Effects of nebulized morphine sulfate on the exercise tolerance of the ventilatory limited COPD patient. Chest 1993;104(1):175–8. - PubMed
Bruera 2005 {published data only}
-
- Bruera E, Sala R, Spruyt O, Palmer L, Zhang T, Willey J. Nebulized versus subcutaneous morphine for patients with cancer dyspnea: a preliminary study. Journal of Pain and Symptom Management 2005;29(6):613‐8. - PubMed
Navigante 2003 {published data only}
-
- Navigante AH, Castro M, Cerchietti L. Morphine plus midazolam versus oxygen therapy on severe dyspnea management in the last week of life in hipoxemic advanced cancer patients [Morfina más midazolan versus oxigenoterapia en el control de la disnea severa durante la última semana de vida en pacientes hipoxémicos con cáncer avanzado]. Medicina Paliativa 2003;10(1):14‐9.
Peterson 1996 {published data only}
-
- Peterson GM, Young RS, Dunne PF, Galloway JG, Parks TE. Pilot study of nebulised morphine for dyspnoea in palliative care patients. Australian Journal of Hospital Pharmacy 1996;26(5):545–7.
Shorati 2012 {published data only}
Smith 2009 {published data only}
-
- Smith TJ, Coyne P, French W, Ramakrishnan V, Corrigan P. Failure to accrue to a study of nebulized fentanyl for dyspnea: lessons learned. Journal of Palliative Medicine 2009;12(9):771‐2. - PubMed
Thomas 2010 {published data only}
-
- Thomas J, Stambler N, Israel RJ. Methylnaltrexone, a peripheral opioid antagonist for opioid‐induced constipation in advanced illness: an analysis of dyspnea in patients with chronic obstructive pulmonary disease (COPD) in two double‐blind randomized trials. 18th International Congress on Palliative Care; Oct 5‐8, 2010; Montreal. Journal of Palliative Care 2010;26(3):242.
References to ongoing studies
Cuervo Pinna 2012 {published data only}
-
- Cuervo Pinna MA. A randomized cross‐over clinical trial to evaluate the efficacy of oral transmucosal fentanyl citrate in the treatment of dyspnea on exertion in patients with advanced cancer. Palliative Medicine 2012;26(4):569‐70. - PubMed
Daubert 2014 {published data only}
-
- Daubert E, Bolesta S. Effect of lorazepam versus morphine on quality of life in hospice patients with dyspnea and anxiety. Journal of the American Pharmacists Association 2014;54(2):e193.
Additional references
ATS 1999
-
- American Thoracic Society. Dyspnea. Mechanisms, assessment, and management: a consensus statement. Amercan Journal of Respiratory and Critical Care Medicine 1999;159(1):321‐40. - PubMed
Banzett 2000
-
- Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activate the insular cortex. NeuroReport 2000;11(10):2117‐20. - PubMed
Bausewein 2008
Beach 2006
-
- Beach D, Schwartzstein RM. The genesis of breathlessness ‐ what do we understand?. In: Booth S, Dudgeon D editor(s). Dyspnoea in Advanced Disease ‐ A Guide to Clinical Management. 1st Edition. Oxford: Oxford University Press, 2006:1‐18.
Bolsher 1987
-
- Bolser DC, Lindsey BG, Shannon R. Medullary inspiratory activity: influence of intercostal tendon organs and muscle spindle endings. Journal of Applied Physiology 1987;62(3):1046‐56. - PubMed
Bolsher 1988
-
- Bolser DC, Lindsey BG, Shannon R. Respiratory pattern changes produced by intercostal muscle/rib vibration. Journal of Applied Physiology 1988;64(6):2458‐62. - PubMed
Brannan 2001
Chahl 1996
-
- Chahl LA. Opioids ‐ mechanisms of action. Australian Prescriber 1996;19:63‐5.
Cranston 2008
Currow 2011
-
- Currow DC, McDonald C, Oaten S, Kenny B, Allcroft P, Frith P, et al. Once‐daily opioids for chronic dyspnea: a dose increment and pharmacovigilance study. Journal of Pain and Symptom Management 2011;42(3):388‐99. - PubMed
Dechartres 2013
Dudgeon 1998
-
- Dudgeon J, Lertzman M. Dyspnea in the advanced cancer patient. Journal of Pain and Symptom Management 1998;16(4):212‐9. - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Evans 2002
-
- Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. Journal of Neurophysiology 2002;88(3):1500‐11. - PubMed
Fitzgerald 1986
-
- Fitzgerald RS, Lahiri S. Reflex response to chemoreceptor stimulation. In: Cherniack NS, Widdicombe JG editor(s). Handbook of Physiology, Section 3: The Respiratory System, Vol. 2. Control of Breathing. Bethesda: American Physiological Society, 1986:313‐62.
GradePro 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org. GRADEpro Guideline Development Tool [Software]. McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org, 2015.
Guz 1997
-
- Guz A. Brain, breathing and breathlessness. Respiration Physiology 1997;109(3):197‐204. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Liberati 2009
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of Internal Medicine 2009;151(4):W65‐94. - PubMed
Liotti 2001
Mahler 2010
-
- Mahler DA, Selecky PA, Harrod CG, Benditt JO, Carrieri‐Kohlman V, Curtis JR, et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest 2010;137(3):674‐91. - PubMed
Mahler 2013
-
- Mahler DA. Opioids for refractory dyspnoea. Expert Review of Respiratory Medicine 2013;7(2):123‐35. - PubMed
Manning 1995
-
- Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. The New England Journal of Medicine 1995;333(23):1547‐53. - PubMed
Masood 1996
-
- Masood AR, Thomas SH. Systemic absorption of nebulized morphine compared with oral morphine in healthy subjects. British Journal of Clinical Pharmacology 1996;41(3):250‐2. - PubMed
McGavin 1978
Nattie 1995
-
- Nattie E. Central chemoreceptors. In: Dempsey JA, Pack AL editor(s). Regulation of Breathing. 2nd Edition. New York: Marcel Dekker, 1995:473‐510.
Neuman 2006
-
- Neuman A, Gunnbjörnsdottir M, Tunsäter A, Nyström L, Franklin KA, Norrman E, et al. Dyspnea in relation to symptoms of anxiety and depression: a prospective population study. Respiratory Medicine 2006;100(10):1843‐9. - PubMed
Nishino 2011
-
- Nishino T. Dyspnoea: underlying mechanisms and treatment. British Journal of Anaesthesia 2011;106(4):463‐74. - PubMed
Nüesch 2010
O'Donnell 1998
-
- O’Donnell DE, L.M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158(5):1557–65. - PubMed
Oxberry 2012
-
- Oxberry S, Jones L, Clark AL, Johnson MJ. Attitudes to morphine in chronic heart failure patients. Postgraduate Medical Journal 2012;88(1043):515‐21. - PubMed
Parshall 2012
Parsons 2001
Pattinson 2009
Peiffer 2001
-
- Peiffer C, Poline JB, Thivard L, Aubier M, Samson Y. Neural substrates for the perception of acutely induced dyspnea. American Journal of Respiratory and Critical Care Medicine 2001;163(4):951‐7. - PubMed
Petrovic 2002
-
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia‐‐ imaging a shared neuronal network. Science 2002;295(5560):1737‐40. - PubMed
Polosa 2002
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rocker 2012
Simon 2010
Uronis 2011
von Leupoldt 2009
-
- Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, et al. Dyspnoea and pain share emotion‐related brain network. NeuroImage 2009;48(1):200‐6. - PubMed
Widdicombe 1982
-
- Widdicombe JG. Pulmonary and respiratory tract receptors. Journal of Experimental Biology 1982;100:42‐57. - PubMed
Wiseman 2013
-
- Wiseman R, Rowett D, Allcroft P, Abernethy A, Currow DC. Chronic refractory dyspnoea‐‐evidence based management. Australian Family Physician 2013;42(3):137‐40. - PubMed
Zebraski 2000
-
- Zebraski SE, Kochenash SM, Raffa RB. Lung opioid receptors: pharmacology and possible target for nebulized morphine in dyspnea. Life Sciences 2000;66(23):2221‐31. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

