B Cell Responses during Secondary Dengue Virus Infection Are Dominated by Highly Cross-Reactive, Memory-Derived Plasmablasts
- PMID: 27030262
- PMCID: PMC4886779
- DOI: 10.1128/JVI.03203-15
B Cell Responses during Secondary Dengue Virus Infection Are Dominated by Highly Cross-Reactive, Memory-Derived Plasmablasts
Abstract
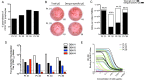
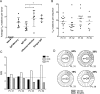
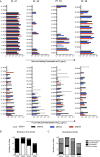
Dengue virus (DENV) infection results in the production of both type-specific and cross-neutralizing antibodies. While immunity to the infecting serotype is long-lived, heterotypic immunity wanes a few months after infection. Epidemiological studies link secondary heterotypic infections with more severe symptoms, and cross-reactive, poorly neutralizing antibodies have been implicated in this increased disease severity. To understand the cellular and functional properties of the acute dengue virus B cell response and its role in protection and immunopathology, we characterized the plasmablast response in four secondary DENV type 2 (DENV2) patients. Dengue plasmablasts had high degrees of somatic hypermutation, with a clear preference for replacement mutations. Clonal expansions were also present in each donor, strongly supporting a memory origin for these acutely induced cells. We generated 53 monoclonal antibodies (MAbs) from sorted patient plasmablasts and found that DENV-reactive MAbs were largely envelope specific and cross neutralizing. Many more MAbs neutralized DENV than reacted to envelope protein, emphasizing the significance of virion-dependent B cell epitopes and the limitations of envelope protein-based antibody screening. A majority of DENV-reactive MAbs, irrespective of neutralization potency, enhanced infection by antibody-dependent enhancement (ADE). Interestingly, even though DENV2 was the infecting serotype in all four patients, several MAbs from two patients neutralized DENV1 more potently than DENV2. Further, half of all type-specific neutralizing MAbs were also DENV1 biased in binding. Taken together, these findings are reminiscent of original antigenic sin (OAS), given that the patients had prior dengue virus exposures. These data describe the ongoing B cell response in secondary patients and may further our understanding of the impact of antibodies in dengue virus pathogenesis.
Importance: In addition to their role in protection, antibody responses have been hypothesized to contribute to the pathology of dengue. Recent studies characterizing memory B cell (MBC)-derived MAbs have provided valuable insight into the targets and functions of B cell responses generated after DENV exposure. However, in the case of secondary infections, such MBC-based approaches fail to distinguish acutely induced cells from the preexisting MBC pool. Our characterization of plasmablasts and plasmablast-derived MAbs provides a focused analysis of B cell responses activated during ongoing infection. Additionally, our studies provide evidence of OAS in the acute-phase dengue virus immune response, providing a basis for future work examining the impact of OAS phenotype antibodies on protective immunity and disease severity in secondary infections.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- WHO. 2009. Dengue guidelines for diagnosis, treatment, prevention and control, new edition. World Health Organization, Geneva, Switzerland. - PubMed
-
- WHO. 2012. Global strategy for dengue prevention and control, 2012-2020. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

