Immunotargeting of Integrin α6β4 for Single-Photon Emission Computed Tomography and Near-Infrared Fluorescence Imaging in a Pancreatic Cancer Model
- PMID: 27030400
- PMCID: PMC5469600
- DOI: 10.1177/1536012115624917
Immunotargeting of Integrin α6β4 for Single-Photon Emission Computed Tomography and Near-Infrared Fluorescence Imaging in a Pancreatic Cancer Model
Abstract
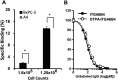
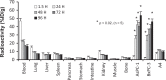
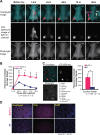
To explore suitable imaging probes for early and specific detection of pancreatic cancer, we demonstrated that α6β4integrin is a good target and employed single-photon emission computed tomography (SPECT) or near-infrared (NIR) imaging for immunotargeting. Expression levels of α6β4were examined by Western blotting and flow cytometry in certain human pancreatic cancer cell lines. The human cell line BxPC-3 was used for α6β4-positive and a mouse cell line, A4, was used for negative counterpart. We labeled antibody against α6β4with Indium-111 ((111)In) or indocyanine green (ICG). After injection of(111)In-labeled probe to tumor-bearing mice, biodistribution, SPECT, autoradiography (ARG), and immunohistochemical (IHC) studies were conducted. After administration of ICG-labeled probe, in vivo and ex vivo NIR imaging and fluorescence microscopy of tumors were performed. BxPC-3 tumor showed a higher radioligand binding in SPECT and higher fluorescence intensity as well as a delay in the probe washout in NIR imaging when compared to A4 tumor. The biodistribution profile of(111)In-labeled probe, ARG, and IHC confirmed the α6β4specific binding of the probe. Here, we propose that α6β4is a desirable target for the diagnosis of pancreatic cancer and that it could be detected by radionuclide imaging and NIR imaging using a radiolabeled or ICG-labeled α6β4antibody.
Keywords: 111In SPECT; ICG; NIR imaging; integrin α6β4; pancreatic cancer.
© The Author(s) 2016.
Conflict of interest statement
Figures


Similar articles
-
Radioimmunotherapy of pancreatic cancer xenografts in nude mice using 90Y-labeled anti-α6β4 integrin antibody.Oncotarget. 2016 Jun 21;7(25):38835-38844. doi: 10.18632/oncotarget.9631. Oncotarget. 2016. PMID: 27246980 Free PMC article.
-
Near-infrared photoimmunotherapy of pancreatic cancer using an indocyanine green-labeled anti-tissue factor antibody.World J Gastroenterol. 2018 Dec 28;24(48):5491-5504. doi: 10.3748/wjg.v24.i48.5491. World J Gastroenterol. 2018. PMID: 30622378 Free PMC article.
-
Micro-positron emission tomography/contrast-enhanced computed tomography imaging of orthotopic pancreatic tumor-bearing mice using the αvβ₃ integrin tracer ⁶⁴Cu-labeled cyclam-RAFT-c(-RGDfK-)₄.Mol Imaging. 2013 Sep;12(6):376-87. Mol Imaging. 2013. PMID: 23981783
-
Molecular Imaging of Pancreatic Cancer with Antibodies.Mol Pharm. 2016 Jan 4;13(1):8-24. doi: 10.1021/acs.molpharmaceut.5b00626. Epub 2015 Dec 10. Mol Pharm. 2016. PMID: 26620581 Free PMC article. Review.
-
In Vivo Molecular Imaging.Biol Pharm Bull. 2017;40(10):1605-1615. doi: 10.1248/bpb.b17-00505. Biol Pharm Bull. 2017. PMID: 28966233 Review.
Cited by
-
Radioimmunotherapy of pancreatic cancer xenografts in nude mice using 90Y-labeled anti-α6β4 integrin antibody.Oncotarget. 2016 Jun 21;7(25):38835-38844. doi: 10.18632/oncotarget.9631. Oncotarget. 2016. PMID: 27246980 Free PMC article.
-
Radioimmunotherapy of Pancreatic Ductal Adenocarcinoma: A Review of the Current Status of Literature.Cancers (Basel). 2020 Feb 19;12(2):481. doi: 10.3390/cancers12020481. Cancers (Basel). 2020. PMID: 32092952 Free PMC article. Review.
-
Molecular Imaging of Pancreatic Duct Adenocarcinoma Using a Type 2 Cannabinoid Receptor-Targeted Near-Infrared Fluorescent Probe.Transl Oncol. 2018 Oct;11(5):1065-1073. doi: 10.1016/j.tranon.2018.06.009. Epub 2018 Jul 9. Transl Oncol. 2018. PMID: 30005208 Free PMC article.
-
Utilizing High Resolution Ultrasound to Monitor Tumor Onset and Growth in Genetically Engineered Pancreatic Cancer Models.J Vis Exp. 2018 Apr 7;(134):56979. doi: 10.3791/56979. J Vis Exp. 2018. PMID: 29683461 Free PMC article.
-
GLI1 activation by non-classical pathway integrin αvβ3/ERK1/2 maintains stem cell-like phenotype of multicellular aggregates in gastric cancer peritoneal metastasis.Cell Death Dis. 2019 Jul 31;10(8):574. doi: 10.1038/s41419-019-1776-x. Cell Death Dis. 2019. PMID: 31366904 Free PMC article.
References
-
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7(3):163–172. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. - PubMed
-
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

