Variation in relapse frequency and the transmission potential of Plasmodium vivax malaria
- PMID: 27030414
- PMCID: PMC4822465
- DOI: 10.1098/rspb.2016.0048
Variation in relapse frequency and the transmission potential of Plasmodium vivax malaria
Abstract
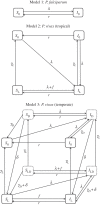
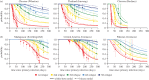
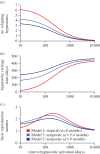
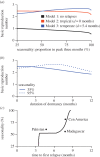
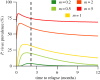
There is substantial variation in the relapse frequency of Plasmodium vivax malaria, with fast-relapsing strains in tropical areas, and slow-relapsing strains in temperate areas with seasonal transmission. We hypothesize that much of the phenotypic diversity in P. vivax relapses arises from selection of relapse frequency to optimize transmission potential in a given environment, in a process similar to the virulence trade-off hypothesis. We develop mathematical models of P. vivax transmission and calculate the basic reproduction number R0 to investigate how transmission potential varies with relapse frequency and seasonality. In tropical zones with year-round transmission, transmission potential is optimized at intermediate relapse frequencies of two to three months: slower-relapsing strains increase the opportunity for onward transmission to mosquitoes, but also increase the risk of being outcompeted by faster-relapsing strains. Seasonality is an important driver of relapse frequency for temperate strains, with the time to first relapse predicted to be six to nine months, coinciding with the duration between seasonal transmission peaks. We predict that there is a threshold degree of seasonality, below which fast-relapsing tropical strains are selected for, and above which slow-relapsing temperate strains dominate, providing an explanation for the observed global distribution of relapse phenotypes.
Keywords: Plasmodium vivax malaria; mathematical model; relapse; seasonality; transmission potential.
© 2016 The Authors.
Figures

Similar articles
-
Modeling Plasmodium vivax: relapses, treatment, seasonality, and G6PD deficiency.J Theor Biol. 2013 Jan 7;316:25-34. doi: 10.1016/j.jtbi.2012.08.024. Epub 2012 Aug 29. J Theor Biol. 2013. PMID: 22959914 Free PMC article.
-
Geographical variation in Plasmodium vivax relapse.Malar J. 2014 Apr 15;13:144. doi: 10.1186/1475-2875-13-144. Malar J. 2014. PMID: 24731298 Free PMC article. Review.
-
Quantifying effect of geographic location on epidemiology of Plasmodium vivax malaria.Emerg Infect Dis. 2013 Jul;19(7):1058-65. doi: 10.3201/eid1907.121674. Emerg Infect Dis. 2013. PMID: 23763820 Free PMC article.
-
Modelling the contribution of the hypnozoite reservoir to Plasmodium vivax transmission.Elife. 2014 Nov 18;3:e04692. doi: 10.7554/eLife.04692. Elife. 2014. PMID: 25406065 Free PMC article.
-
Determinants of relapse periodicity in Plasmodium vivax malaria.Malar J. 2011 Oct 11;10:297. doi: 10.1186/1475-2875-10-297. Malar J. 2011. PMID: 21989376 Free PMC article. Review.
Cited by
-
Superinfection and the hypnozoite reservoir for Plasmodium vivax: a general framework.J Math Biol. 2023 Dec 1;88(1):7. doi: 10.1007/s00285-023-02014-3. J Math Biol. 2023. PMID: 38040981 Free PMC article.
-
The empirical support for the radical cure strategy for eliminating Plasmodium vivax in China.BMC Med. 2022 Jan 21;20(1):17. doi: 10.1186/s12916-021-02214-y. BMC Med. 2022. PMID: 35057816 Free PMC article.
-
Treatment of pigs with endectocides as a complementary tool for combating malaria transmission by Anopheles farauti (s.s.) in Papua New Guinea.Parasit Vectors. 2019 Mar 19;12(1):124. doi: 10.1186/s13071-019-3392-0. Parasit Vectors. 2019. PMID: 30890165 Free PMC article.
-
Population-level estimates of the proportion of Plasmodium vivax blood-stage infections attributable to relapses among febrile patients attending Adama Malaria Diagnostic Centre, East Shoa Zone, Oromia, Ethiopia.Malar J. 2017 Jul 27;16(1):301. doi: 10.1186/s12936-017-1944-3. Malar J. 2017. PMID: 28750669 Free PMC article.
-
Whole genome sequencing of Plasmodium vivax isolates reveals frequent sequence and structural polymorphisms in erythrocyte binding genes.PLoS Negl Trop Dis. 2020 Oct 12;14(10):e0008234. doi: 10.1371/journal.pntd.0008234. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33044985 Free PMC article.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

