Structure Based Docking and Molecular Dynamic Studies of Plasmodial Cysteine Proteases against a South African Natural Compound and its Analogs
- PMID: 27030511
- PMCID: PMC4814779
- DOI: 10.1038/srep23690
Structure Based Docking and Molecular Dynamic Studies of Plasmodial Cysteine Proteases against a South African Natural Compound and its Analogs
Abstract
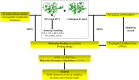
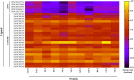
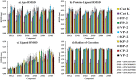
Identification of potential drug targets as well as development of novel antimalarial chemotherapies with unique mode of actions due to drug resistance by Plasmodium parasites are inevitable. Falcipains (falcipain-2 and falcipain-3) of Plasmodium falciparum, which catalyse the haemoglobin degradation process, are validated drug targets. Previous attempts to develop peptide based drugs against these enzymes have been futile due to the poor pharmacological profiles and susceptibility to degradation by host enzymes. This study aimed to identify potential non-peptide inhibitors against falcipains and their homologs from other Plasmodium species. Structure based virtual docking approach was used to screen a small non-peptidic library of natural compounds from South Africa against 11 proteins. A potential hit, 5α-Pregna-1,20-dien-3-one (5PGA), with inhibitory activity against plasmodial proteases and selectivity on human cathepsins was identified. A 3D similarity search on the ZINC database using 5PGA identified five potential hits based on their docking energies. The key interacting residues of proteins with compounds were identified via molecular dynamics and free binding energy calculations. Overall, this study provides a basis for further chemical design for more effective derivatives of these compounds. Interestingly, as these compounds have cholesterol-like nuclei, they and their derivatives might be well tolerated in humans.
Figures








Similar articles
-
South African Abietane Diterpenoids and Their Analogs as Potential Antimalarials: Novel Insights from Hybrid Computational Approaches.Molecules. 2019 Nov 7;24(22):4036. doi: 10.3390/molecules24224036. Molecules. 2019. PMID: 31703388 Free PMC article.
-
Computation-based virtual screening for designing novel antimalarial drugs by targeting falcipain-III: a structure-based drug designing approach.J Vector Borne Dis. 2013 Apr-Jun;50(2):93-102. J Vector Borne Dis. 2013. PMID: 23995310
-
In-Silico molecular docking and simulation studies on novel chalcone and flavone hybrid derivatives with 1, 2, 3-triazole linkage as vital inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase.J Biomol Struct Dyn. 2018 Nov;36(15):3993-4009. doi: 10.1080/07391102.2017.1404935. Epub 2017 Nov 27. J Biomol Struct Dyn. 2018. PMID: 29132266
-
Targeting Cysteine Proteases from Plasmodium falciparum: A General Overview, Rational Drug Design and Computational Approaches for Drug Discovery.Curr Drug Targets. 2018;19(5):501-526. doi: 10.2174/1389450117666161221122432. Curr Drug Targets. 2018. PMID: 28003005 Review.
-
Development of Plasmodium falciparum protease inhibitors in the past decade (2002-2012).Curr Med Chem. 2013;20(25):3049-68. doi: 10.2174/0929867311320250003. Curr Med Chem. 2013. PMID: 23514416 Review.
Cited by
-
Phytoconstituents of Withania somnifera (L.) Dunal (Ashwagandha) unveiled potential cerebroside sulfotransferase inhibitors: insight through virtual screening, molecular dynamics, toxicity, and reverse pharmacophore analysis.J Biol Eng. 2024 Oct 23;18(1):59. doi: 10.1186/s13036-024-00456-x. J Biol Eng. 2024. PMID: 39444022 Free PMC article.
-
South African Abietane Diterpenoids and Their Analogs as Potential Antimalarials: Novel Insights from Hybrid Computational Approaches.Molecules. 2019 Nov 7;24(22):4036. doi: 10.3390/molecules24224036. Molecules. 2019. PMID: 31703388 Free PMC article.
-
Molecular Docking Analysis of Pyrimethamine Derivatives with Plasmodium falciparum Dihydrofolate Reductase.Bioinformation. 2018 May 31;14(5):232-235. doi: 10.6026/97320630014232. eCollection 2018. Bioinformation. 2018. PMID: 30108420 Free PMC article.
-
Target-Based Virtual Screening of Natural Compounds Identifies a Potent Antimalarial With Selective Falcipain-2 Inhibitory Activity.Front Pharmacol. 2022 Apr 6;13:850176. doi: 10.3389/fphar.2022.850176. eCollection 2022. Front Pharmacol. 2022. PMID: 35462917 Free PMC article.
-
Discorhabdin N, a South African Natural Compound, for Hsp72 and Hsc70 Allosteric Modulation: Combined Study of Molecular Modeling and Dynamic Residue Network Analysis.Molecules. 2019 Jan 6;24(1):188. doi: 10.3390/molecules24010188. Molecules. 2019. PMID: 30621342 Free PMC article.
References
-
- Severini C. & Menegon M. Resistance to antimalarial drugs: An endless world war against Plasmodium that we risk losing. J. Glob. Antimicrob. Resist. 3, 58–63 (2015). - PubMed
-
- WHO, Q&A on artemisinin resistance. (2015) Available at: http://who.int/malaria/media/artemisinin_resistance_qa/en/. (Accessed: 8th August 2015).
-
- WHO, World Malaria Report 2013. (2013) Available at: http://www.who.int/malaria/publications/world_malaria_report_2013/wmr201.... (Accessed: 26th July 2015).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

