Production of a Recombinant Dengue Virus 2 NS5 Protein and Potential Use as a Vaccine Antigen
- PMID: 27030586
- PMCID: PMC4895003
- DOI: 10.1128/CVI.00081-16
Production of a Recombinant Dengue Virus 2 NS5 Protein and Potential Use as a Vaccine Antigen
Abstract
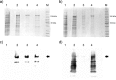
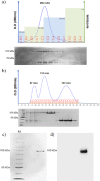
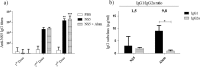
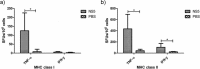
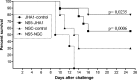
Dengue fever is caused by any of the four known dengue virus serotypes (DENV1 to DENV4) that affect millions of people worldwide, causing a significant number of deaths. There are vaccines based on chimeric viruses, but they still are not in clinical use. Anti-DENV vaccine strategies based on nonstructural proteins are promising alternatives to those based on whole virus or structural proteins. The DENV nonstructural protein 5 (NS5) is the main target of anti-DENV T cell-based immune responses in humans. In this study, we purified a soluble recombinant form of DENV2 NS5 expressed in Escherichia coli at large amounts and high purity after optimization of expression conditions and purification steps. The purified DENV2 NS5 was recognized by serum from DENV1-, DENV2-, DENV3-, or DENV4-infected patients in an epitope-conformation-dependent manner. In addition, immunization of BALB/c mice with NS5 induced high levels of NS5-specific antibodies and expansion of gamma interferon- and tumor necrosis factor alpha-producing T cells. Moreover, mice immunized with purified NS5 were partially protected from lethal challenges with the DENV2 NGC strain and with a clinical isolate (JHA1). These results indicate that the recombinant NS5 protein preserves immunological determinants of the native protein and is a promising vaccine antigen capable of inducing protective immune responses.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Tetravalent recombinant dengue virus-like particles as potential vaccine candidates: immunological properties.BMC Microbiol. 2014 Dec 18;14:233. doi: 10.1186/s12866-014-0233-3. BMC Microbiol. 2014. PMID: 25520151 Free PMC article.
-
Recombinant dengue 2 virus NS3 protein conserves structural antigenic and immunological properties relevant for dengue vaccine design.Virus Genes. 2014 Oct;49(2):185-95. doi: 10.1007/s11262-014-1087-3. Epub 2014 May 23. Virus Genes. 2014. PMID: 24854144
-
Antibody Epitopes Identified in Critical Regions of Dengue Virus Nonstructural 1 Protein in Mouse Vaccination and Natural Human Infections.J Immunol. 2017 May 15;198(10):4025-4035. doi: 10.4049/jimmunol.1700029. Epub 2017 Apr 5. J Immunol. 2017. PMID: 28381638 Free PMC article.
-
[Research progress in the structure and function of dengue virus non-structural 1 protein].Bing Du Xue Bao. 2014 Nov;30(6):683-8. Bing Du Xue Bao. 2014. PMID: 25868284 Review. Chinese.
-
Identification and selection of immunodominant B and T cell epitopes for dengue multi-epitope-based vaccine.Med Microbiol Immunol. 2021 Feb;210(1):1-11. doi: 10.1007/s00430-021-00700-x. Epub 2021 Jan 30. Med Microbiol Immunol. 2021. PMID: 33515283 Review.
Cited by
-
Adaptive Immunity to Dengue Virus: Slippery Slope or Solid Ground for Rational Vaccine Design?Pathogens. 2020 Jun 15;9(6):470. doi: 10.3390/pathogens9060470. Pathogens. 2020. PMID: 32549226 Free PMC article. Review.
-
Dengue Virus Non-Structural Protein 5 as a Versatile, Multi-Functional Effector in Host-Pathogen Interactions.Front Cell Infect Microbiol. 2021 Mar 18;11:574067. doi: 10.3389/fcimb.2021.574067. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33816326 Free PMC article. Review.
-
An Overview of Current Uses and Future Opportunities for Computer-Assisted Design of Vaccines for Neglected Tropical Diseases.Adv Appl Bioinform Chem. 2021 Feb 15;14:25-47. doi: 10.2147/AABC.S258759. eCollection 2021. Adv Appl Bioinform Chem. 2021. PMID: 33623396 Free PMC article. Review.
-
Humoral immune response induced with dengue virus-like particles serotypes 1 and 4 produced in silkworm.AMB Express. 2022 Jan 31;12(1):8. doi: 10.1186/s13568-022-01353-6. AMB Express. 2022. PMID: 35102445 Free PMC article.
-
Optimization and scale-up production of Zika virus ΔNS1 in Escherichia coli: application of Response Surface Methodology.AMB Express. 2019 Dec 31;10(1):1. doi: 10.1186/s13568-019-0926-y. AMB Express. 2019. PMID: 31893321 Free PMC article.
References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi:10.1038/nature12060. - DOI - PMC - PubMed
-
- Blok J. 1985. Genetic relationships of the dengue virus serotypes. J Gen Virol 66:1323–1325. - PubMed
-
- Chan YC, Kanapathipillai K, Chew KS. 1965. Isolation of two strains of dengue virus type 3 in Singapore. Singapore Med J 5:127–132. - PubMed
-
- Diercks FH. 1959. Isolation of a type 2 dengue virus by use of hamster kidney cell cultures. Am J Trop Med Hyg 8:488–491. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

