Somatic activating mutations in Pik3ca cause sporadic venous malformations in mice and humans
- PMID: 27030595
- PMCID: PMC5973268
- DOI: 10.1126/scitranslmed.aad9982
Somatic activating mutations in Pik3ca cause sporadic venous malformations in mice and humans
Abstract
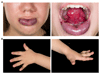
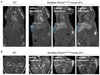
Venous malformations (VMs) are painful and deforming vascular lesions composed of dilated vascular channels, which are present from birth. Mutations in the TEK gene, encoding the tyrosine kinase receptor TIE2, are found in about half of sporadic (nonfamilial) VMs, and the causes of the remaining cases are unknown. Sclerotherapy, widely accepted as first-line treatment, is not fully efficient, and targeted therapy for this disease remains underexplored. We have generated a mouse model that faithfully mirrors human VM through mosaic expression of Pik3ca(H1047R), a constitutively active mutant of the p110α isoform of phosphatidylinositol 3-kinase (PI3K), in the embryonic mesoderm. Endothelial expression of Pik3ca(H1047R)resulted in endothelial cell (EC) hyperproliferation, reduction in pericyte coverage of blood vessels, and decreased expression of arteriovenous specification markers. PI3K pathway inhibition with rapamycin normalized EC hyperproliferation and pericyte coverage in postnatal retinas and stimulated VM regression in vivo. In line with the mouse data, we also report the presence of activating PIK3CA mutations in human VMs, mutually exclusive with TEK mutations. Our data demonstrate a causal relationship between activating Pik3ca mutations and the genesis of VMs, provide a genetic model that faithfully mirrors the normal etiology and development of this human disease, and establish the basis for the use of PI3K-targeted therapies in VMs.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
B.V. is consultant to Karus Therapeutics (Oxford, UK). M.F.L. serves as a board member of the International Society for Magnetic Resonance in Medicine (British Chapter). All other authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- G0901338/MRC_/Medical Research Council/United Kingdom
- MC_UP_1502/1/MRC_/Medical Research Council/United Kingdom
- 097721/Z/11/Z/WT_/Wellcome Trust/United Kingdom
- FS/15/33/31608/BHF_/British Heart Foundation/United Kingdom
- MC_UU_12012/5/MRC_/Medical Research Council/United Kingdom
- 098498/WT_/Wellcome Trust/United Kingdom
- BBS/B/15481/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 097721/WT_/Wellcome Trust/United Kingdom
- WT098498/WT_/Wellcome Trust/United Kingdom
- C23338/A15965/CRUK_/Cancer Research UK/United Kingdom
- 15965/CRUK_/Cancer Research UK/United Kingdom
- A15965/CRUK_/Cancer Research UK/United Kingdom
- WT104076MA/WT_/Wellcome Trust/United Kingdom
- 104076/WT_/Wellcome Trust/United Kingdom
- MRC_MC_UU_12012/5/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

