The role of the rectum in osmoregulation and the potential effect of renoguanylin on SLC26a6 transport activity in the Gulf toadfish (Opsanus beta)
- PMID: 27030664
- PMCID: PMC4967237
- DOI: 10.1152/ajpregu.00033.2016
The role of the rectum in osmoregulation and the potential effect of renoguanylin on SLC26a6 transport activity in the Gulf toadfish (Opsanus beta)
Abstract
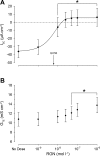
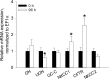
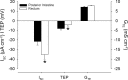
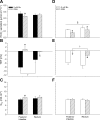
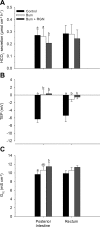
Teleosts living in seawater continually absorb water across the intestine to compensate for branchial water loss to the environment. The present study reveals that the Gulf toadfish (Opsanus beta) rectum plays a comparable role to the posterior intestine in ion and water absorption. However, the posterior intestine appears to rely more on SLC26a6 (a HCO3 (-)/Cl(-) antiporter) and the rectum appears to rely on NKCC2 (SLC12a1) for the purposes of solute-coupled water absorption. The present study also demonstrates that the rectum responds to renoguanylin (RGN), a member of the guanylin family of peptides that alters the normal osmoregulatory processes of the distal intestine, by inhibited water absorption. RGN decreases rectal water absorption more greatly than in the posterior intestine and leads to net Na(+) and Cl(-) secretion, and a reversal of the absorptive short-circuit current (ISC). It is hypothesized that maintaining a larger fluid volume within the distal segments of intestinal tract facilitates the removal of CaCO3 precipitates and other solids from the intestine. Indeed, the expression of the components of the Cl(-)-secretory response, apical CFTR, and basolateral NKCC1 (SLC12a2), are upregulated in the rectum of the Gulf toadfish after 96 h in 60 ppt, an exposure that increases CaCO3 precipitate formation relative to 35 ppt. Moreover, the downstream intracellular effects of RGN appear to directly inhibit ion absorption by NKCC2 and anion exchange by SLC26a6. Overall, the present findings elucidate key electrophysiological differences between the posterior intestine and rectum of Gulf toadfish and the potent regulatory role renoguanylin plays in osmoregulation.
Keywords: CFTR; Cl− secretion; HCO3− secretion; intestine; marine teleost; water secretion.
Copyright © 2016 the American Physiological Society.
Figures
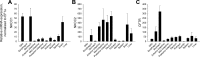




Similar articles
-
The differential role of renoguanylin in osmoregulation and apical Cl-/HCO3- exchange activity in the posterior intestine of the Gulf toadfish (Opsanus beta).Am J Physiol Regul Integr Comp Physiol. 2015 Aug 15;309(4):R399-409. doi: 10.1152/ajpregu.00118.2015. Epub 2015 May 27. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26017493
-
Guanylin peptides regulate electrolyte and fluid transport in the Gulf toadfish (Opsanus beta) posterior intestine.Am J Physiol Regul Integr Comp Physiol. 2014 Nov 1;307(9):R1167-79. doi: 10.1152/ajpregu.00188.2014. Epub 2014 Aug 6. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 25100079
-
Renoguanylin stimulates apical CFTR translocation and decreases HCO3- secretion through PKA activity in the Gulf toadfish (Opsanus beta).J Exp Biol. 2018 Mar 26;221(Pt 6):jeb173948. doi: 10.1242/jeb.173948. J Exp Biol. 2018. PMID: 29361605
-
Intestinal anion exchange in marine teleosts is involved in osmoregulation and contributes to the oceanic inorganic carbon cycle.Acta Physiol (Oxf). 2011 Jul;202(3):421-34. doi: 10.1111/j.1748-1716.2010.02241.x. Epub 2011 Mar 1. Acta Physiol (Oxf). 2011. PMID: 21362153 Review.
-
The intestinal guanylin system and seawater adaptation in eels.Gen Comp Endocrinol. 2007 Jun-Jul;152(2-3):339-51. doi: 10.1016/j.ygcen.2007.05.005. Epub 2007 May 13. Gen Comp Endocrinol. 2007. PMID: 17561018 Review.
Cited by
-
Osmoregulatory contributions of the corticotropin-releasing factor system in the intestine of Atlantic salmon.J Exp Biol. 2025 Jul 15;228(14):jeb250052. doi: 10.1242/jeb.250052. Epub 2025 May 15. J Exp Biol. 2025. PMID: 40123464 Free PMC article.
-
Nuclear Factor of Activated T Cells-5 Regulates Notochord Lumenogenesis in Chordate Larval Development.Int J Mol Sci. 2022 Nov 19;23(22):14407. doi: 10.3390/ijms232214407. Int J Mol Sci. 2022. PMID: 36430885 Free PMC article.
-
Na+/HCO3- cotransporter 1 (nbce1) isoform gene expression during smoltification and seawater acclimation of Atlantic salmon.J Comp Physiol B. 2022 Sep;192(5):577-592. doi: 10.1007/s00360-022-01443-8. Epub 2022 Jun 17. J Comp Physiol B. 2022. PMID: 35715660
-
Osmoregulation by the gastro-intestinal tract of marine fish at depth - implications for the global carbon cycle.J Exp Biol. 2025 Jul 15;228(14):jeb249834. doi: 10.1242/jeb.249834. Epub 2025 Jul 18. J Exp Biol. 2025. PMID: 40679146 Free PMC article.
-
Dietary Marginal and Excess Selenium Increased Triglycerides Deposition, Induced Endoplasmic Reticulum Stress and Differentially Influenced Selenoproteins Expression in the Anterior and Middle Intestines of Yellow Catfish Pelteobagrus fulvidraco.Antioxidants (Basel). 2021 Mar 29;10(4):535. doi: 10.3390/antiox10040535. Antioxidants (Basel). 2021. PMID: 33805536 Free PMC article.
References
-
- Ando M, Nagashima K. Intestinal Na+ and Cl− levels control drinking behavior in the seawater-adapted eel Anguilla japonica. J Exp Biol 199: 711–716, 1996. - PubMed
-
- Ando M, Takei Y. Guanylin activates Cl−1 secretion into the lumen of seawater eel intestine via apical Cl− channel under simulated in vivo conditions. Am J Physiol Regul Integr Comp Physiol 308: R400–R410, 2015. - PubMed
-
- Ando M, Wong MK, Takei Y. Mechanisms of guanylin action on water and ion absorption at different regions of seawater eel intestine. Am J Physiol Regul Integr Comp Physiol 307: R653–R663, 2014. - PubMed
-
- Arshad N, Visweswariah SS. Cyclic nucleotide signaling in intestinal epithelia: getting to the gut of the matter. Wiley Interdiscip Rev Syst Biol Med 5: 409–424, 2013. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

