New and emerging targeted therapies for cystic fibrosis
- PMID: 27030675
- PMCID: PMC4817245
- DOI: 10.1136/bmj.i859
New and emerging targeted therapies for cystic fibrosis
Abstract
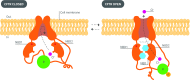
Cystic fibrosis (CF) is a monogenic autosomal recessive disorder that affects about 70,000 people worldwide. The clinical manifestations of the disease are caused by defects in the cystic fibrosis transmembrane conductance regulator (CFTR) protein. The discovery of the CFTR gene in 1989 has led to a sophisticated understanding of how thousands of mutations in the CFTR gene affect the structure and function of the CFTR protein. Much progress has been made over the past decade with the development of orally bioavailable small molecule drugs that target defective CFTR proteins caused by specific mutations. Furthermore, there is considerable optimism about the prospect of gene replacement or editing therapies to correct all mutations in cystic fibrosis. The recent approvals of ivacaftor and lumacaftor represent the genesis of a new era of precision medicine in the treatment of this condition. These drugs are having a positive impact on the lives of people with cystic fibrosis and are potentially disease modifying. This review provides an update on advances in our understanding of the structure and function of the CFTR, with a focus on state of the art targeted drugs that are in development.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following: BSQ has had no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; SMR has received travel reimbursements to attend investigators’ meetings held by Vertex Pharmaceuticals, Novartis, Bayer Healthcare, PTC Therapeutics. SMR has an unlicensed patent held by the University of Alabama Birmingham on the use of CFTR activators for the treatment of respiratory diseases unaffected by acquired or genetic causes of CFTR dysfunction. SMR has an unlicensed patent held by the University of Alabama Birmingham for the use of optical coherence tomography as a diagnostic tool.
Figures



Similar articles
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2020 Dec 17;12(12):CD010966. doi: 10.1002/14651858.CD010966.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Nov 20;11:CD010966. doi: 10.1002/14651858.CD010966.pub4. PMID: 33331662 Free PMC article. Updated.
-
Lumacaftor alone and combined with ivacaftor: preclinical and clinical trial experience of F508del CFTR correction.Expert Rev Respir Med. 2016;10(1):5-17. doi: 10.1586/17476348.2016.1122527. Epub 2015 Dec 9. Expert Rev Respir Med. 2016. PMID: 26581802
-
[New therapies for cystic fibrosis targeting the CFTR gene or the CFTR protein].Rev Mal Respir. 2016 Oct;33(8):658-665. doi: 10.1016/j.rmr.2015.11.010. Epub 2016 Jan 21. Rev Mal Respir. 2016. PMID: 26806675 Review. French.
-
A new era in the treatment of cystic fibrosis.Clin Med (Lond). 2014 Feb;14(1):76-8. doi: 10.7861/clinmedicine.14-1-76. Clin Med (Lond). 2014. PMID: 24532752 Free PMC article. No abstract available.
-
Cystic fibrosis transmembrane conductance regulator-modifying medications: the future of cystic fibrosis treatment.Ann Pharmacother. 2012 Jul-Aug;46(7-8):1065-75. doi: 10.1345/aph.1R076. Epub 2012 Jun 26. Ann Pharmacother. 2012. PMID: 22739718 Review.
Cited by
-
Cystic Fibrosis Related Diabetes: a Unique Challenge in Diabetes Care.Mo Med. 2016 Sep-Oct;113(5):384-389. Mo Med. 2016. PMID: 30228505 Free PMC article.
-
Unraveling the CFTR Function-Phenotype Connection for Precision Treatment in Cystic Fibrosis.Am J Respir Crit Care Med. 2019 May 1;199(9):1053-1054. doi: 10.1164/rccm.201903-0696ED. Am J Respir Crit Care Med. 2019. PMID: 30939246 Free PMC article. No abstract available.
-
Protein folding: Illuminating chaperone activity.Nat Chem Biol. 2017 Mar 22;13(4):346-347. doi: 10.1038/nchembio.2332. Nat Chem Biol. 2017. PMID: 28328919 No abstract available.
-
The burden of cystic fibrosis in North Africa.Front Genet. 2024 Jan 10;14:1295008. doi: 10.3389/fgene.2023.1295008. eCollection 2023. Front Genet. 2024. PMID: 38269366 Free PMC article. Review.
-
New drugs in cystic fibrosis: what has changed in the last decade?Ther Adv Chronic Dis. 2022 May 21;13:20406223221098136. doi: 10.1177/20406223221098136. eCollection 2022. Ther Adv Chronic Dis. 2022. PMID: 35620188 Free PMC article. Review.
References
-
- WHO. Genomic Resource Centre. Genes and human disease. http://www.who.int/genomics/public/geneticdiseases/en/index2.html.
-
- Macneill SJ. Hodson and Geddes’ cystic fibrosis. Epidemiology of cystic fibrosis. 4th ed CRC Press, 2015.
-
- Bear CE, Li CH, Kartner N, et al. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell 1992;68:809-18. - PubMed
-
- Rich DP, Anderson MP, Gregory RJ, et al. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature 1990;347:358-63. - PubMed
-
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005;352:1992-2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
