Functional characterization of a mouse model for central post-stroke pain
- PMID: 27030713
- PMCID: PMC4956143
- DOI: 10.1177/1744806916629049
Functional characterization of a mouse model for central post-stroke pain
Abstract
Background: Stroke patients often suffer from a central neuropathic pain syndrome called central post-stroke pain. This syndrome is characterized by evoked pain hypersensitivity as well as spontaneous, on-going pain in the body area affected by the stroke. Clinical evidence strongly suggests a dysfunction in central pain pathways as an important pathophysiological factor in the development of central post-stroke pain, but the exact underlying mechanisms remain poorly understood. To elucidate the underlying pathophysiology of central post-stroke pain, we generated a mouse model that is based on a unilateral stereotactic lesion of the thalamic ventral posterolateral nucleus, which typically causes central post-stroke pain in humans.
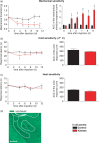
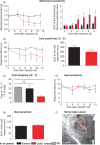
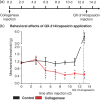
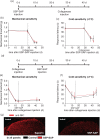
Results: Behavioral analysis showed that the sensory changes in our model are comparable to the sensory abnormalities observed in patients suffering from central post-stroke pain. Surprisingly, pharmacological inhibition of spinal and peripheral key components of the pain system had no effect on the induction or maintenance of the evoked hypersensitivity observed in our model. In contrast, microinjection of lidocaine into the thalamic lesion completely reversed injury-induced hypersensitivity.
Conclusions: These results suggest that the evoked hypersensitivity observed in central post-stroke pain is causally linked to on-going neuronal activity in the lateral thalamus.
Keywords: central post-stroke pain; mouse model; pain; stroke.
© The Author(s) 2016.
Figures

Similar articles
-
A new central post-stroke pain rat model: autologous blood injected thalamic hemorrhage involved increased expression of P2X4 receptor.Neurosci Lett. 2018 Nov 20;687:124-130. doi: 10.1016/j.neulet.2018.09.023. Epub 2018 Sep 26. Neurosci Lett. 2018. PMID: 30267847
-
Development and pharmacological verification of a new mouse model of central post-stroke pain.Neurosci Res. 2014 Jan;78:72-80. doi: 10.1016/j.neures.2013.09.005. Epub 2013 Sep 19. Neurosci Res. 2014. PMID: 24055601
-
Late-onset hypersensitivity after a lesion in the ventral posterolateral nucleus of the thalamus: A macaque model of central post-stroke pain.Sci Rep. 2017 Sep 4;7(1):10316. doi: 10.1038/s41598-017-10679-2. Sci Rep. 2017. PMID: 28871156 Free PMC article.
-
Central post-stroke pain: clinical characteristics, pathophysiology, and management.Lancet Neurol. 2009 Sep;8(9):857-68. doi: 10.1016/S1474-4422(09)70176-0. Lancet Neurol. 2009. PMID: 19679277 Review.
-
Plasticity of pain-related neuronal activity in the human thalamus.Prog Brain Res. 2006;157:353-64. doi: 10.1016/S0079-6123(06)57021-9. Prog Brain Res. 2006. PMID: 17046675 Review.
Cited by
-
Non-invasive Brain Stimulation for Central Neuropathic Pain.Front Mol Neurosci. 2022 May 19;15:879909. doi: 10.3389/fnmol.2022.879909. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35663263 Free PMC article. Review.
-
Microglial depletion under thalamic hemorrhage ameliorates mechanical allodynia and suppresses aberrant axonal sprouting.JCI Insight. 2020 Feb 13;5(3):e131801. doi: 10.1172/jci.insight.131801. JCI Insight. 2020. PMID: 32051342 Free PMC article.
-
Dexmedetomidine attenuates haemorrhage-induced thalamic pain by inhibiting the TLR4/NF-κB/ERK1/2 pathway in mice.Inflammopharmacology. 2021 Dec;29(6):1751-1760. doi: 10.1007/s10787-021-00877-w. Epub 2021 Oct 13. Inflammopharmacology. 2021. PMID: 34643849 Free PMC article.
-
Central Post-Stroke Pain: An Integrative Review of Somatotopic Damage, Clinical Symptoms, and Neurophysiological Measures.Front Neurol. 2021 Aug 18;12:678198. doi: 10.3389/fneur.2021.678198. eCollection 2021. Front Neurol. 2021. PMID: 34484097 Free PMC article.
-
Methods Used to Evaluate Pain Behaviors in Rodents.Front Mol Neurosci. 2017 Sep 6;10:284. doi: 10.3389/fnmol.2017.00284. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28932184 Free PMC article.
References
-
- Bowsher D. Pain after thalamic stroke: right diencephalic predominance and clinical features in 180 patients. Neurology 1998; 51: 927. - PubMed
-
- MacGowan DJ, Janal MN, Clark WC, et al. Central poststroke pain and Wallenberg’s lateral medullary infarction: frequency, character, and determinants in 63 patients. Neurology 1997; 49: 120–125. - PubMed
-
- Andersen G, Vestergaard K, Ingeman-Nielsen M, et al. Incidence of central post-stroke pain. Pain 1995; 61: 187–193. - PubMed
-
- Appelros P. Prevalence and predictors of pain and fatigue after stroke: a population-based study. Int J Rehab Res 2006; 29: 329–333. DOI: 10.1097/MRR.0b013e328010c7b8. - PubMed
-
- Bowsher D. Stroke and central poststroke pain in an elderly population. J Pain 2001; 2: 258–261. DOI: 10.1054/jpai.2001.24549. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

