Receptive field size, chemical and thermal responses, and fiber conduction velocity of rat chorda tympani geniculate ganglion neurons
- PMID: 27030734
- PMCID: PMC4946609
- DOI: 10.1152/jn.00045.2016
Receptive field size, chemical and thermal responses, and fiber conduction velocity of rat chorda tympani geniculate ganglion neurons
Abstract
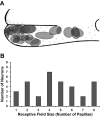
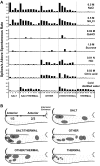
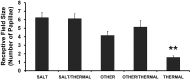
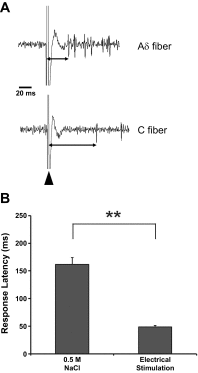
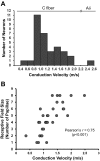
Afferent chorda tympani (CT) fibers innervating taste and somatosensory receptors in fungiform papillae have neuron cell bodies in the geniculate ganglion (GG). The GG/CT fibers branch in the tongue to innervate taste buds in several fungiform papillae. To investigate receptive field characteristics of GG/CT neurons, we recorded extracellular responses from GG cells to application of chemical and thermal stimuli. Receptive field size was mapped by electrical stimulation of individual fungiform papillae. Response latency to electrical stimulation was used to determine fiber conduction velocity. Responses of GG neurons to lingual application of stimuli representing four taste qualities, and water at 4°C, were used to classify neuron response properties. Neurons classified as SALT, responding only to NaCl and NH4Cl, had a mean receptive field size of six papillae. Neurons classified as OTHER responded to salts and other chemical stimuli and had smaller mean receptive fields of four papillae. Neurons that responded to salts and cold stimuli, classified as SALT/THERMAL, and neurons responding to salts, other chemical stimuli and cold, classified as OTHER/THERMAL, had mean receptive field sizes of six and five papillae, respectively. Neurons responding only to cold stimuli, categorized as THERMAL, had receptive fields of one to two papillae located at the tongue tip. Based on conduction velocity most of the neurons were classified as C fibers. Neurons with large receptive fields had higher conduction velocities than neurons with small receptive fields. These results demonstrate that GG neurons can be distinguished by receptive field size, response properties and afferent fiber conduction velocity derived from convergent input of multiple taste organs.
Keywords: chemosensory; chorda tympani; geniculate ganglion; taste; taste bud.
Copyright © 2016 the American Physiological Society.
Figures


Similar articles
-
Geniculate Ganglion Neurons are Multimodal and Variable in Receptive Field Characteristics.Neuroscience. 2017 Dec 26;367:147-158. doi: 10.1016/j.neuroscience.2017.10.032. Epub 2017 Oct 31. Neuroscience. 2017. PMID: 29097269
-
Developmental decrease in size of peripheral receptive fields of single chorda tympani nerve fibers and relation to increasing NaCl taste sensitivity.J Neurosci. 1988 Jan;8(1):64-72. doi: 10.1523/JNEUROSCI.08-01-00064.1988. J Neurosci. 1988. PMID: 3339419 Free PMC article.
-
Taste-responsive neurons of the glossopharyngeal nerve of the rat.J Neurophysiol. 1991 Jun;65(6):1452-63. doi: 10.1152/jn.1991.65.6.1452. J Neurophysiol. 1991. PMID: 1875254
-
Cracking taste codes by tapping into sensory neuron impulse traffic.Prog Neurobiol. 2008 Nov;86(3):245-63. doi: 10.1016/j.pneurobio.2008.09.003. Epub 2008 Sep 7. Prog Neurobiol. 2008. PMID: 18824076 Free PMC article. Review.
-
Recognizing Taste: Coding Patterns Along the Neural Axis in Mammals.Chem Senses. 2019 Apr 15;44(4):237-247. doi: 10.1093/chemse/bjz013. Chem Senses. 2019. PMID: 30788507 Free PMC article. Review.
Cited by
-
Biphasic functions for the GDNF-Ret signaling pathway in chemosensory neuron development and diversification.Proc Natl Acad Sci U S A. 2018 Jan 16;115(3):E516-E525. doi: 10.1073/pnas.1708838115. Epub 2017 Dec 27. Proc Natl Acad Sci U S A. 2018. PMID: 29282324 Free PMC article.
-
Cell non-autonomous requirement of p75 in the development of geniculate oral sensory neurons.Sci Rep. 2020 Dec 17;10(1):22117. doi: 10.1038/s41598-020-78816-y. Sci Rep. 2020. PMID: 33335119 Free PMC article.
-
Anterior and Posterior Tongue Regions and Taste Papillae: Distinct Roles and Regulatory Mechanisms with an Emphasis on Hedgehog Signaling and Antagonism.Int J Mol Sci. 2023 Mar 2;24(5):4833. doi: 10.3390/ijms24054833. Int J Mol Sci. 2023. PMID: 36902260 Free PMC article. Review.
-
Advances in Optical Tools to Study Taste Sensation.Mol Cells. 2022 Dec 31;45(12):877-882. doi: 10.14348/molcells.2022.0116. Epub 2022 Dec 13. Mol Cells. 2022. PMID: 36572557 Free PMC article. Review.
-
Transcriptomes and neurotransmitter profiles of classes of gustatory and somatosensory neurons in the geniculate ganglion.Nat Commun. 2017 Oct 2;8(1):760. doi: 10.1038/s41467-017-01095-1. Nat Commun. 2017. PMID: 28970527 Free PMC article.
References
-
- Abe J, Hosokawa H, Okazawa M, Kandachi M, Sawada Y, Yamanaka K, Matsumura K, Kobayashi S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Mol Brain Res 136: 91–98, 2005. - PubMed
-
- Beidler LM. Innervation of rat fungiform papilla. In: Olfaction and Taste III, edited by Pfaffmann C. New York: Rockefeller University Press, 1969, p. 352–369.
-
- Boudreau JC, Bradley BE, Bierer PR, Kruger S, Tsuchitani C. Single unit recordings from the geniculate ganglion of the facial nerve of the cat. Exp Brain Res 13: 461–488, 1971. - PubMed
-
- Boudreau JC, Sivakumar L, Do LT, White TD, Oravec J, Hoang NK. Neurophysiology of geniculate ganglion (facial nerve) taste systems: species comparisons. Chem Senses 10: 89–128, 1985.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

