EGCG Inhibited Lipofuscin Formation Based on Intercepting Amyloidogenic β-Sheet-Rich Structure Conversion
- PMID: 27030967
- PMCID: PMC4816542
- DOI: 10.1371/journal.pone.0152064
EGCG Inhibited Lipofuscin Formation Based on Intercepting Amyloidogenic β-Sheet-Rich Structure Conversion
Abstract
Background: Lipofuscin (LF) is formed during lipid peroxidation and sugar glycosylation by carbonyl-amino crosslinks with biomacrolecules, and accumulates slowly within postmitotic cells. The environmental pollution, modern dietary culture and lifestyle changes have been found to be the major sources of reactive carbonyl compounds in vivo. Irreversible carbonyl-amino crosslinks induced by carbonyl stress are essentially toxiferous for aging-related functional losses in modern society. Results show that (-)-epigallocatechin gallate (EGCG), the main polyphenol in green tea, can neutralize the carbonyl-amino cross-linking reaction and inhibit LF formation, but the underlying mechanism is unknown.
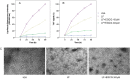
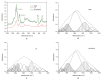
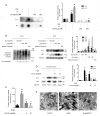
Methods and results: We explored the mechanism of the neutralization process from protein, cell, and animal levels using spectrofluorometry, infrared spectroscopy, conformation antibodies, and electron microscopy. LF demonstrated an amyloidogenic β-sheet-rich with antiparallel structure, which accelerated the carbonyl-amino crosslinks formation and disrupted proteolysis in both PC12 cells and D-galactose (D-gal)-induced brain aging mice models. Additionally, EGCG effectively inhibited the formation of the amyloidogenic β-sheet-rich structure of LF, and prevented its conversion into toxic and on-pathway aggregation intermediates, thereby cutting off the carbonyl-amino crosslinks.
Conclusions: Our study indicated that the amyloidogenic β-sheet structure of LF may be the core driving force for carbonyl-amino crosslinks further formation, which mediates the formation of amyloid fibrils from native state of biomacrolecules. That EGCG exhibits anti-amyloidogenic β-sheet-rich structure properties to prevent the LF formation represents a novel strategy to impede the development of degenerative processes caused by ageing or stress-induced premature senescence in modern environments.
Conflict of interest statement
Figures

Similar articles
-
Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (-)-epigallocatechin-3-gallate.FASEB J. 2003 May;17(8):952-4. doi: 10.1096/fj.02-0881fje. Epub 2003 Mar 28. FASEB J. 2003. PMID: 12670874
-
(-)-epigallocatechin-3-gallate (EGCG) maintains kappa-casein in its pre-fibrillar state without redirecting its aggregation pathway.J Mol Biol. 2009 Sep 25;392(3):689-700. doi: 10.1016/j.jmb.2009.07.031. Epub 2009 Jul 17. J Mol Biol. 2009. PMID: 19616561
-
Green Tea Polyphenol Microparticles Based on the Oxidative Coupling of EGCG Inhibit Amyloid Aggregation/Cytotoxicity and Serve as a Platform for Drug Delivery.ACS Biomater Sci Eng. 2020 Aug 10;6(8):4414-4423. doi: 10.1021/acsbiomaterials.0c00188. Epub 2020 Jul 9. ACS Biomater Sci Eng. 2020. PMID: 33455167
-
An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration.Front Neurosci. 2018 Jul 5;12:464. doi: 10.3389/fnins.2018.00464. eCollection 2018. Front Neurosci. 2018. PMID: 30026686 Free PMC article. Review.
-
The Essential Mechanisms of Aging: What Have We Learnt in Ten Years?Curr Top Med Chem. 2016;16(5):503-10. doi: 10.2174/1568026615666150813142854. Curr Top Med Chem. 2016. PMID: 26268334 Review.
Cited by
-
Natural polyphenols as novel interventions for aging and age-related diseases: Exploring efficacy, mechanisms of action and implications for future research.Chin Herb Med. 2024 Sep 3;17(2):279-291. doi: 10.1016/j.chmed.2024.09.001. eCollection 2025 Apr. Chin Herb Med. 2024. PMID: 40256718 Free PMC article. Review.
-
Pathophysiological Response to SARS-CoV-2 Infection Detected by Infrared Spectroscopy Enables Rapid and Robust Saliva Screening for COVID-19.Biomedicines. 2022 Feb 1;10(2):351. doi: 10.3390/biomedicines10020351. Biomedicines. 2022. PMID: 35203562 Free PMC article.
-
Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer's Disease.Int J Mol Sci. 2019 Oct 14;20(20):5090. doi: 10.3390/ijms20205090. Int J Mol Sci. 2019. PMID: 31615073 Free PMC article. Review.
-
Inhibitory Effects of Six Types of Tea on Aging and High-Fat Diet-Related Amyloid Formation Activities.Antioxidants (Basel). 2021 Sep 24;10(10):1513. doi: 10.3390/antiox10101513. Antioxidants (Basel). 2021. PMID: 34679648 Free PMC article.
References
-
- Yin DZ. The essential mechanisms of aging: what have we learnt in ten years? Curr Top Med Chem. 2015; 16 (5): 503–510. - PubMed
-
- Pevroux J, Sternberg M. Advanced glycation end products (AGEs): pharmacological inhibition in diabetes. Pathol Biol. 2006; 54 (7): 405–419. - PubMed
-
- Yin DZ. Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Radical Bio Med. 1996; 21(6): 871–888. - PubMed
-
- Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003; 25(3–4): 207–218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

