PI3Kγ Is Critical for Dendritic Cell-Mediated CD8+ T Cell Priming and Viral Clearance during Influenza Virus Infection
- PMID: 27030971
- PMCID: PMC4816423
- DOI: 10.1371/journal.ppat.1005508
PI3Kγ Is Critical for Dendritic Cell-Mediated CD8+ T Cell Priming and Viral Clearance during Influenza Virus Infection
Abstract
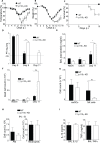
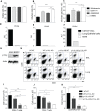
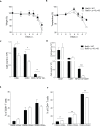
Phosphoinositide-3-kinases have been shown to be involved in influenza virus pathogenesis. They are targeted directly by virus proteins and are essential for efficient viral replication in infected lung epithelial cells. However, to date the role of PI3K signaling in influenza infection in vivo has not been thoroughly addressed. Here we show that one of the PI3K subunits, p110γ, is in fact critically required for mediating the host's antiviral response. PI3Kγ deficient animals exhibit a delayed viral clearance and increased morbidity during respiratory infection with influenza virus. We demonstrate that p110γ is required for the generation and maintenance of potent antiviral CD8+ T cell responses through the developmental regulation of pulmonary cross-presenting CD103+ dendritic cells under homeostatic and inflammatory conditions. The defect in lung dendritic cells leads to deficient CD8+ T cell priming, which is associated with higher viral titers and more severe disease course during the infection. We thus identify PI3Kγ as a novel key host protective factor in influenza virus infection and shed light on an unappreciated layer of complexity concerning the role of PI3K signaling in this context.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
DNGR-1 is dispensable for CD8+ T-cell priming during respiratory syncytial virus infection.Eur J Immunol. 2014 Aug;44(8):2340-8. doi: 10.1002/eji.201444454. Epub 2014 May 30. Eur J Immunol. 2014. PMID: 24777856
-
Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells.J Immunol. 2011 Dec 1;187(11):6011-21. doi: 10.4049/jimmunol.1100987. Epub 2011 Oct 31. J Immunol. 2011. PMID: 22043017
-
Potential role of invariant NKT cells in the control of pulmonary inflammation and CD8+ T cell response during acute influenza A virus H3N2 pneumonia.J Immunol. 2011 May 15;186(10):5590-602. doi: 10.4049/jimmunol.1002348. Epub 2011 Apr 13. J Immunol. 2011. PMID: 21490153
-
Deficiency of the NOD-Like Receptor NLRC5 Results in Decreased CD8+ T Cell Function and Impaired Viral Clearance.J Virol. 2017 Aug 10;91(17):e00377-17. doi: 10.1128/JVI.00377-17. Print 2017 Sep 1. J Virol. 2017. PMID: 28615208 Free PMC article.
-
Phosphatidyl Inositol 3 Kinase-Gamma Balances Antiviral and Inflammatory Responses During Influenza A H1N1 Infection: From Murine Model to Genetic Association in Patients.Front Immunol. 2018 May 15;9:975. doi: 10.3389/fimmu.2018.00975. eCollection 2018. Front Immunol. 2018. PMID: 29867955 Free PMC article.
Cited by
-
Frontline Science: Coincidental null mutation of Csf2rα in a colony of PI3Kγ-/- mice causes alveolar macrophage deficiency and fatal respiratory viral infection.J Leukoc Biol. 2017 Feb;101(2):367-376. doi: 10.1189/jlb.4HI0316-157R. Epub 2016 Jul 28. J Leukoc Biol. 2017. PMID: 27468760 Free PMC article.
-
Traumatic brain injury alters dendritic cell differentiation and distribution in lymphoid and non-lymphoid organs.J Neuroinflammation. 2022 Oct 1;19(1):238. doi: 10.1186/s12974-022-02609-5. J Neuroinflammation. 2022. PMID: 36183126 Free PMC article.
-
PI3Kγ Regulatory Protein p84 Determines Mast Cell Sensitivity to Ras Inhibition-Moving Towards Cell Specific PI3K Targeting?Front Immunol. 2020 Oct 28;11:585070. doi: 10.3389/fimmu.2020.585070. eCollection 2020. Front Immunol. 2020. PMID: 33193405 Free PMC article.
-
Function, Regulation and Biological Roles of PI3Kγ Variants.Biomolecules. 2019 Aug 30;9(9):427. doi: 10.3390/biom9090427. Biomolecules. 2019. PMID: 31480354 Free PMC article. Review.
-
Conserved sequence features in intracellular domains of viral spike proteins.Virology. 2024 Nov;599:110198. doi: 10.1016/j.virol.2024.110198. Epub 2024 Aug 2. Virology. 2024. PMID: 39116647
References
-
- Zvelebil MJ, MacDougall L, Leevers S, Volinia S, Vanhaesebroeck B, Gout I, et al. Structural and functional diversity of phosphoinositide 3-kinases. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1996;351(1336):217–23. 10.1098/rstb.1996.0019 . - DOI - PubMed
-
- Hiles ID, Otsu M, Volinia S, Fry MJ, Gout I, Dhand R, et al. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992;70(3):419–29. . - PubMed
-
- Otsu M, Hiles I, Gout I, Fry MJ, Ruiz-Larrea F, Panayotou G, et al. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991;65(1):91–104. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

