The Principal Forces of Oocyte Polarity Are Evolutionary Conserved but May Not Affect the Contribution of the First Two Blastomeres to the Blastocyst Development in Mammals
- PMID: 27030988
- PMCID: PMC4816511
- DOI: 10.1371/journal.pone.0148382
The Principal Forces of Oocyte Polarity Are Evolutionary Conserved but May Not Affect the Contribution of the First Two Blastomeres to the Blastocyst Development in Mammals
Abstract
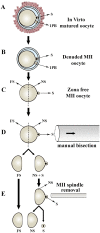
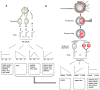
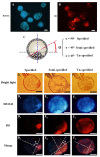
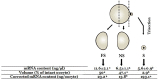
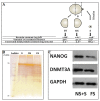
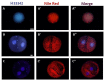
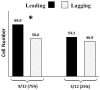
Oocyte polarity and embryonic patterning are well-established features of development in lower species. Whether a similar form of pre-patterning exists in mammals is currently under hot debate in mice. This study investigated this issue for the first time in ovine as a large mammal model. Microsurgical trisection of unfertilized MII-oocytes revealed that cortical cytoplasm around spindle (S) contained significant amounts of total maternal mRNAs and proteins compared to matched cytoplast hemispheres that were located either near (NS) or far (FS) -to-spindle. RT-qPCR provided striking examples of maternal mRNA localized to subcellular substructures S (NPM2, GMNN, H19, PCAF, DNMT3A, DNMT1, and STELLA), NS (SOX2, NANOG, POU5F1, and TET1), and FS (GCN) of MII oocyte. Immunoblotting revealed that specific maternal proteins DNMT3A and NANOG were asymmetrically enriched in MII-spindle-half of the oocytes. Topological analysis of sperm entry point (SEP) revealed that sperm preferentially entered via the MII-spindle-half of the oocytes. Even though, the topological position of first cleavage plane with regard to SEP was quite stochastic. Spatial comparison of lipid content revealed symmetrical distribution of lipids between 2-cell blastomeres. Lineage tracing using Dil, a fluorescent dye, revealed that while the progeny of leading blastomere of 2-cell embryos contributed to more cells in the developed blastocysts compared to lagging counterpart, the contributions of leading and lagging blastomeres to the embryonic-abembryonic parts of the developed blastocysts were almost unbiased. And finally, separated sister blastomeres of 2-cell embryos had an overall similar probability to arrest at any stage before the blastocyst (2-cell, 4-cell, 8-cell, and morula) or to achieve the blastocyst stage. It was concluded that the localization of maternal mRNAs and proteins at the spindle are evolutionarily conserved between mammals unfertilized ovine oocyte could be considered polar with respect to the spatial regionalization of maternal transcripts and proteins. Even though, the principal forces of this definitive oocyte polarity may not persist during embryonic cleavages.
Conflict of interest statement
Figures


Similar articles
-
Evidence of Oocyte Polarity in Bovine; Implications for Intracytoplasmic Sperm Injection and Somatic Cell Nuclear Transfer.Cell J. 2017 Oct;19(3):482-491. doi: 10.22074/cellj.2017.4887. Epub 2017 Aug 19. Cell J. 2017. PMID: 28836411 Free PMC article.
-
Retrospective analysis: reproducibility of interblastomere differences of mRNA expression in 2-cell stage mouse embryos is remarkably poor due to combinatorial mechanisms of blastomere diversification.Mol Hum Reprod. 2018 Jul 1;24(7):388-400. doi: 10.1093/molehr/gay021. Mol Hum Reprod. 2018. PMID: 29746690
-
Unbiased contribution of the first two blastomeres to mouse blastocyst development.Mol Reprod Dev. 2005 Nov;72(3):354-61. doi: 10.1002/mrd.20353. Mol Reprod Dev. 2005. PMID: 16078274
-
Spatial arrangement of individual 4-cell stage blastomeres and the order in which they are generated correlate with blastocyst pattern in the mouse embryo.Mech Dev. 2005 Apr;122(4):487-500. doi: 10.1016/j.mod.2004.11.014. Epub 2004 Dec 18. Mech Dev. 2005. PMID: 15804563
-
[Recent contributions to the establishment of the axes of the mammalian embryo].Morphologie. 2002 Jun;86(273):5-8. Morphologie. 2002. PMID: 12224392 Review. French.
Cited by
-
Perfect date-the review of current research into molecular bases of mammalian fertilization.J Assist Reprod Genet. 2020 Feb;37(2):243-256. doi: 10.1007/s10815-019-01679-4. Epub 2020 Jan 6. J Assist Reprod Genet. 2020. PMID: 31909446 Free PMC article. Review.
-
Totipotency segregates between the sister blastomeres of two-cell stage mouse embryos.Sci Rep. 2017 Aug 15;7(1):8299. doi: 10.1038/s41598-017-08266-6. Sci Rep. 2017. PMID: 28811525 Free PMC article.
-
Fertilization and Cleavage Axes Differ In Primates Conceived By Conventional (IVF) Versus Intracytoplasmic Sperm Injection (ICSI).Sci Rep. 2019 Oct 25;9(1):15282. doi: 10.1038/s41598-019-51815-4. Sci Rep. 2019. PMID: 31653971 Free PMC article.
-
Embryo cell allocation patterns are not altered by biopsy but can be linked with further development.Reproduction. 2017 Dec;154(6):807-814. doi: 10.1530/REP-17-0514. Epub 2017 Sep 29. Reproduction. 2017. PMID: 28971891 Free PMC article.
-
Random Allocation of Blastomere Descendants to the Trophectoderm and ICM of the Bovine Blastocyst.Biol Reprod. 2016 Dec;95(6):123. doi: 10.1095/biolreprod.116.141200. Epub 2016 Oct 19. Biol Reprod. 2016. PMID: 27760750 Free PMC article.
References
-
- Edwards RG, Beard HK. Oocyte polarity and cell determination in early mammalian embryos. Molecular Human Reproduction. 1997; 3: 863–905. - PubMed
-
- Gardner R. The early blastocyst is bilaterally symmetrical and its axis of symmetry is aligned with the animal-vegetal axis of the zygote in the mouse. Development. 1997; 124: 289–301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

