ECM hydrogel for the treatment of stroke: Characterization of the host cell infiltrate
- PMID: 27031811
- PMCID: PMC4893791
- DOI: 10.1016/j.biomaterials.2016.03.014
ECM hydrogel for the treatment of stroke: Characterization of the host cell infiltrate
Abstract
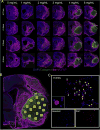
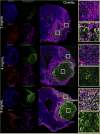
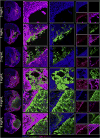
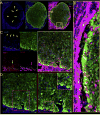
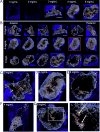
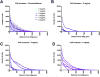
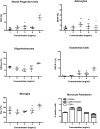
Brain tissue loss following stroke is irreversible with current treatment modalities. The use of an acellular extracellular matrix (ECM), formulated to produce a hydrogel in situ within the cavity formed by a stroke, was investigated as a method to replace necrotic debris and promote the infiltration of host brain cells. Based on magnetic resonance imaging measurements of lesion location and volume, different concentrations of ECM (0, 1, 2, 3, 4, 8 mg/mL) were injected at a volume equal to that of the cavity (14 days post-stroke). Retention of ECM within the cavity occurred at concentrations >3 mg/mL. A significant cell infiltration into the ECM material in the lesion cavity occurred with an average of ∼36,000 cells in the 8 mg/mL concentration within 24 h. An infiltration of cells with distances of >1500 μm into the ECM hydrogel was observed, but the majority of cells were at the tissue/hydrogel boundary. Cells were typically of a microglia, macrophage, or neural and oligodendrocyte progenitor phenotype. At the 8 mg/mL concentration, ∼60% of infiltrating cells were brain-derived phenotypes and 30% being infiltrating peripheral macrophages, polarizing toward an M2-like anti-inflammatory phenotype. These results suggest that an 8 mg/mL ECM concentration promotes a significant acute endogenous repair response that could potentially be exploited to treat stroke.
Keywords: Biomaterial; Brain; Delivery; Extracellular matrix; Hydrogel; Injection; Macrophage; Magnetic resonance imaging; Neural progenitor; Phenotypes; Stereotactic; Stroke.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no personal financial or institutional conflict of interest in any of the drugs, materials, or devices described in this article.
Figures



References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. - PubMed
-
- Dalise S, Ambrosio F, Modo M. Brain plasticity and recovery in preclinical models of stroke. Arch Ital Biol. 2014;152:190–215. - PubMed
-
- Modo M, Ambrosio F, Friedlander RM, Badylak SF, Wechsler LR. Bioengineering solutions for neural repair and recovery in stroke. Curr Opin Neurol. 2013;26:626–631. - PubMed
-
- Smith EJ, Stroemer RP, Gorenkova N, Nakajima M, Crum WR, Tang E, et al. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells. 2012;30:785–796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

