Randomised phase III trial of FEC120 vs EC-docetaxel in patients with high-risk node-positive primary breast cancer: final survival analysis of the ADEBAR study
- PMID: 27031854
- PMCID: PMC4984804
- DOI: 10.1038/bjc.2016.82
Randomised phase III trial of FEC120 vs EC-docetaxel in patients with high-risk node-positive primary breast cancer: final survival analysis of the ADEBAR study
Abstract
Background: Taxane-containing adjuvant chemotherapy has been established as standard treatment in node-positive breast cancer. This study compared efficacy and tolerability of epirubicin (E)/cyclophosphamide (C) followed by docetaxel (Doc) with a dose-dense 5-fluorouracil (F)+E+ C regimen.
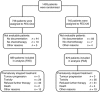
Methods: The ADEBAR study was a randomised phase III trial for women with primary invasive breast cancer and ⩾4 metastatic axillary lymph nodes (n=1364). Treatment consisted of four 21-day cycles of E plus C, followed by four 21-day cycles of Doc (EC-Doc), or six 28-day cycles of E plus F plus C (FEC120).
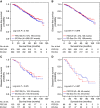
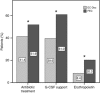
Results: Disease-free survival (DFS) was similar in the two treatment arms as shown by multivariate Cox regression adjusted for other prognostic factors (EC-Doc vs FEC120, hazard ratio (HR): 1.087; 95% confidence interval (CI): 0.878-1.346, P=0.444). In addition, there was no significant difference in overall survival (OS) between the two groups (HR: 0.974; 95% CI: 0.750-1.264, P=0.841). Haematologic toxicity was more common in FEC120 recipients; non-haematologic toxicities occurred more frequently in the EC-Doc arm. The serious adverse event rate was significantly higher in the FEC120 group (29.7% vs 22.5%).
Conclusions: EC-Doc provides a feasible and effective alternative therapy option to FEC120 with a different safety profile in this high-risk breast cancer cohort.
Conflict of interest statement
WJ received research grants from Novartis, GSK, Amgen, Eisai, Roche, Teva, Pierre Fabre, and Janssen Diagnostics. BR received research grants from Sanofi-Aventis, Astra-Zeneca, Amgen, and Novartis and is on the advisory board of Amgen and Novartis. JH is on the advisory board of Sanofi-Aventis.
Figures
References
-
- Abe O, Abe R, Enomoto K, Kikuchi K, Koyama H, Masuda H, Nomura Y, Sakai K, Sugimachi K, Tominaga T, Uchino J, Yoshida M, Haybittle JL, Davies C, Harvey VJ, Holdaway TM, Kay RG, Mason BH, Forbes JF, Wilcken N, Gnant M, Jakesz R, Ploner M, Yosef HMA, Focan C, Lobelle JP, Peek U, Oates GD, Powell J, Durand M, Mauriac L, Di Leo A, Dolci S, Piccart MJ, Masood MB, Parker D, Price JJ, PSGJ Hupperets, Jackson S, Ragaz J, Berry D, Broadwater G, Cirrincione C, Muss H, Norton L, Weiss RB, Abu-Zahra HT, Portnoj SM, Baum M, Cuzick J, Houghton J, Riley D, Gordon NH, Davis HL, Beatrice A, Mihura J, Naja A, Lehingue Y, Romestaing P, Dubois JB, Delozier T, Mace-Lesec'h J, Rambert P, Andrysek O, Barkmanova J, Owen JR, Meier P, Howell A, Ribeiro GC, Swindell R, Alison R, Boreham J, Clarke M, Collins R, Darby S, Elphinstone P, Evans V, Godwin J, Gray R, Harwood C, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Mead G, Peto R, Wang Y, Albano J, de Oliveira CF, Gervasio H, Gordilho J, Johansen H, Mouridsen HT, Gelman RS, Harris JR, Henderson IC, Shapiro CL, Andersen KW, Axelsson CK, Blichert-Toft M, Moller S, Overgaard J, Overgaard M, Rose C, Cartensen B, Palshof T, Trampisch HJ, Dalesio O, de Vries EGE, Rodenhuis S, van Tinteren H, Comis RL, Davidson NE, Robert N, Sledge G, Tormey DC, Wood W, Cameron D, Chetty U, Forrest P, Jack W, Rossbach J, Klijn JGM, Treurniet-Donker AD, van Putten WLJ, Costa A, Veronesi U, Bartelink H, Duchateau L, Legrand C, Sylvester R, van der Hage JA, van de Velde CJH, Cunningham MP, Catalano R, Creech RH, Bonneterre J, Fargeot P, Fumoleau P, Kerbrat P, Namer M, Jonat W, Kaufmann M, Schumacher M, von Minckwitz G, Bastert G, Rauschecker H, Sauer R, Sauerbrei W, Schauer A, de Schryver A, Vakaet L, Belfiglio M, Nicolucci A, Pellegrini F, Sacco M, Valentini M, McArdle CS, Smith DC, Galligioni E, Boccardo F, Rubagotti A, Dent DM, Gudgeon CA, Hacking A, Erazo A, Medina JY, Izuo M, Morishita Y, Takei H, Fentiman IS, Hayward JL, Rubens RD, Skilton D, Graeff H, Janicke F, Meisner C, Scheurlen H, von Fournier D, Dafni U, Fountzilas G, Klefstrom P, Blomqvist C, Saarto T, Margreiter R, Asselain B, Salmon RJ, Vilcoq JR, Arriagada R, Hill C, Laplanche A, Le MG, Spielmann M, Bruzzi P, Montanaro E, Rosso R, Sertoli MR, Venturini M, Amadori D, Benraadt J, Kooi M, van de Velde AO, van Dongen JA, Vermorken JB, Castiglione M, Cavalli F, Coates A, Collins J, Gelber RD, Goldhirsch A, Lindtner J, Price KN, Rudenstam CM, Senn HJ, Bliss JM, Chilvers CED, Coombes RC, Hall E, Marty M, Borovik R, Brufman G, Hayat H, Robinson E, Wigler N, Bonadonna G, Camerini T, De Palo G, Del Vecchio M, Formelli F, Valagussa P, Martoni A, Pannuti F, Cocconi G, Colozza A, Camisa R, Aogi K, Takashima S, Ikeda T, Inokuchi K, Sawa K, Sonoo H, Korzeniowski S, Skolyszewski J, Ogawa M, Yamashita J, Bonte J Early Breast Cancer Trialists' Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365: 1687–1717. - PubMed
-
- Albert JM, Buzdar AU, Guzman R, Allen PK, Strom Ea, Perkins GH, Woodward Wa, Hoffman KE, Tereffe W, Hunt KK, Buchholz Ta, Oh JL (2011) Prospective randomized trial of 5-fluorouracil, doxorubicin, and cyclophosphamide (FAC) vs paclitaxel and FAC (TFAC) in patients with operable breast cancer: Impact of taxane chemotherapy on locoregional control. Breast Cancer Res Treat 128: 421–427. - PubMed
-
- Burnell M, Levine MN, Chapman JW, Bramwell V, Gelmon K, Walley B, Vandenberg T, Chalchal H, Albain KS, Perez EA, Rugo H, Pritchard K, Brien PO, Shepherd LE (2010) Cyclophosphamide, epirubicin, and fluorouracil vs dose-dense epirubicin and cyclophosphamide followed by paclitaxel vs doxorubicin and cyclophosphamide followed by paclitaxel in node-positive or high-risk node-negative breast cancer. J Clin Oncol 28: 77–82. - PMC - PubMed
-
- Clarke M (2006) Meta-analyses of adjuvant therapies for women with early breast cancer: The Early Breast Cancer Trialists' Collaborative Group overview. Ann Oncol 17: 59–62. - PubMed
-
- Coudert B, Asselain B, Campone M, Spielmann M, Machiels J-P, Pénault-Llorca F, Serin D, Lévy C, Romieu G, Canon J-L, Orfeuvre H, Piot G, Petit T, Jerusalem G, Audhuy B, Veyret C, Beauduin M, Eymard J-C, Martin A-L, Roché H (2012) Extended benefit from sequential administration of docetaxel after standard fluorouracil, epirubicin, and cyclophosphamide regimen for node-positive breast cancer: the 8-year follow-up results of the UNICANCER-PACS01 trial. Oncologist 17: 900–909. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

