Pdcd4 restrains the self-renewal and white-to-beige transdifferentiation of adipose-derived stem cells
- PMID: 27031966
- PMCID: PMC4823969
- DOI: 10.1038/cddis.2016.75
Pdcd4 restrains the self-renewal and white-to-beige transdifferentiation of adipose-derived stem cells
Abstract
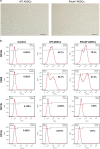
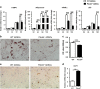
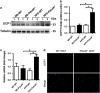
The stemness maintenance of adipose-derived stem cells (ADSCs) is important for adipose homeostasis and energy balance. Programmed cell death 4 (Pdcd4) has been demonstrated to be involved in the development of obesity, but its possible roles in ADSC function and adipogenic capacity remain unclear. In this study, we demonstrate that Pdcd4 is a key controller that limits the self-renewal and white-to-beige transdifferentiation of ADSCs. Pdcd4 deficiency in mice caused stemness enhancement of ADSCs as evidenced by increased expression of CD105, CD90, Nanog and Oct4 on ADSCs, together with enhanced in situ proliferation in adipose tissues. Pdcd4 deficiency promoted proliferation, colony formation of ADSCs and drove more ADSCs entering the S phase accompanied by AKT activation and cyclinD1 upregulation. Blockade of AKT signaling in Pdcd4-deficient ADSCs led to a marked decline in cyclinD1, S-phase entry and cell proliferation, revealing AKT as a target for repressing ADSC self-renewal by Pdcd4. Intriguingly, depletion of Pdcd4 promoted the transdifferentiation of ADSCs into beige adipocytes. A reduction in lipid contents and expression levels of white adipocyte markers including C/EBPα, PPAR-γ, adiponectin and αP2 was detected in Pdcd4-deficient ADSCs during white adipogenic differentiation, substituted by typical beige adipocyte characteristics including small, multilocular lipid droplets and UCP1 expression. More lactate produced by Pdcd4-deficient ADSCs might be an important contributor to the expression of UCP1 and white-to-beige transdifferentiation. In addition, an elevation of UCP1 expression was confirmed in white adipose tissues from Pdcd4-deficient mice upon high-fat diet, which displayed increased energy expenditure and resistance to obesity as compared with wild-type obese mice. These findings provide evidences that Pdcd4 produces unfavorable influences on ADSC stemness, which contribute to adipose dysfunction, obesity and metabolic syndromes, thereby proposing Pdcd4 as a potential intervening target for regulating ADSC function.
Figures




Similar articles
-
Multipotency and cardiomyogenic potential of human adipose-derived stem cells from epicardium, pericardium, and omentum.Stem Cell Res Ther. 2016 Jun 13;7(1):84. doi: 10.1186/s13287-016-0343-y. Stem Cell Res Ther. 2016. PMID: 27296220 Free PMC article.
-
CD90 serves as differential modulator of subcutaneous and visceral adipose-derived stem cells by regulating AKT activation that influences adipose tissue and metabolic homeostasis.Stem Cell Res Ther. 2019 Nov 28;10(1):355. doi: 10.1186/s13287-019-1459-7. Stem Cell Res Ther. 2019. PMID: 31779686 Free PMC article.
-
ZBTB16 Overexpression Enhances White Adipogenesis and Induces Brown-Like Adipocyte Formation of Bovine White Intramuscular Preadipocytes.Cell Physiol Biochem. 2018;48(6):2528-2538. doi: 10.1159/000492697. Epub 2018 Aug 17. Cell Physiol Biochem. 2018. PMID: 30121655
-
Transcriptional regulation of the uncoupling protein-1 gene.Biochimie. 2017 Mar;134:86-92. doi: 10.1016/j.biochi.2016.09.017. Epub 2016 Oct 5. Biochimie. 2017. PMID: 27693079 Review.
-
Role of PRDM16 in the activation of brown fat programming. Relevance to the development of obesity.Histol Histopathol. 2013 Nov;28(11):1411-25. doi: 10.14670/HH-28.1411. Epub 2013 Jun 17. Histol Histopathol. 2013. PMID: 23771475 Review.
Cited by
-
High Expression of a Cancer Stemness-Related Gene, Chromobox 8 (CBX8), in Normal Tissue Adjacent to the Tumor (NAT) Is Associated with Poor Prognosis of Colorectal Cancer Patients.Cells. 2022 Jun 6;11(11):1852. doi: 10.3390/cells11111852. Cells. 2022. PMID: 35681547 Free PMC article.
-
Pdcd4 Is Involved in the Formation of Stress Granule in Response to Oxidized Low-Density Lipoprotein or High-Fat Diet.PLoS One. 2016 Jul 25;11(7):e0159568. doi: 10.1371/journal.pone.0159568. eCollection 2016. PLoS One. 2016. PMID: 27454120 Free PMC article.
-
Microglial Pdcd4 deficiency mitigates neuroinflammation-associated depression via facilitating Daxx mediated PPARγ/IL-10 signaling.J Neuroinflammation. 2024 May 31;21(1):143. doi: 10.1186/s12974-024-03142-3. J Neuroinflammation. 2024. PMID: 38822367 Free PMC article.
-
Navigating the Adipocyte Precursor Niche: Cell-Cell Interactions, Regulatory Mechanisms and Implications for Adipose Tissue Homeostasis.J Cell Signal. 2024;5(2):65-86. doi: 10.33696/signaling.5.114. J Cell Signal. 2024. PMID: 38826152 Free PMC article.
-
Lactate Fluxes and Plasticity of Adipose Tissues: A Redox Perspective.Front Physiol. 2021 Jun 30;12:689747. doi: 10.3389/fphys.2021.689747. eCollection 2021. Front Physiol. 2021. PMID: 34276410 Free PMC article. Review.
References
-
- Baglioni S, Francalanci M, Squecco R, Lombardi A, Cantini G, Angeli R et al. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J 2009; 23: 3494–3505. - PubMed
-
- De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003; 174: 101–109. - PubMed
-
- Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 2012; 21: 2724–2752. - PubMed
-
- Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012; 30: 804–810. - PubMed
-
- Taha MF, Hedayati V. Isolation, identification and multipotential differentiation of mouse adipose tissue-derived stem cells. Tissue Cell 2010; 42: 211–216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

