Bioactivation of Heterocyclic Aromatic Amines by UDP Glucuronosyltransferases
- PMID: 27032077
- PMCID: PMC4868641
- DOI: 10.1021/acs.chemrestox.6b00046
Bioactivation of Heterocyclic Aromatic Amines by UDP Glucuronosyltransferases
Abstract
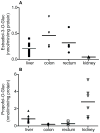
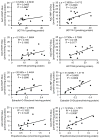
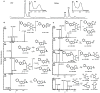
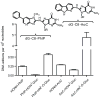
2-Amino-9H-pyrido[2,3-b]indole (AαC) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) are carcinogenic heterocyclic aromatic amines (HAA) that arise during the burning of tobacco and cooking of meats. UDP-glucuronosyltransferases (UGT) detoxicate many procarcinogens and their metabolites. The genotoxic N-hydroxylated metabolite of AαC, 2-hydroxyamino-9H-pyrido[2,3-b]indole (HONH-AαC), undergoes glucuronidation to form the isomeric glucuronide (Gluc) conjugates N(2)-(β-d-glucosidurony1)-2-hydroxyamino-9H-pyrido[2,3-b]indole (AαC-HON(2)-Gluc) and O-(β-d-glucosidurony1)-2-hydroxyamino-9H-pyrido[2,3-b]indole (AαC-HN(2)-O-Gluc). AαC-HON(2)-Gluc is a stable metabolite but AαC-HN(2)-O-Gluc is a biologically reactive intermediate, which covalently adducts to DNA at levels that are 20-fold higher than HONH-AαC. We measured the rates of formation of AαC-HON(2)-Gluc and AαC-HN(2)-O-Gluc in human organs: highest activity occurred with liver and kidney microsomes, and lesser activity was found with colon and rectum microsomes. AαC-HN(2)-O-Gluc formation was largely diminished in liver and kidney microsomes, by niflumic acid, a selective inhibitor UGT1A9. In contrast, AαC-HON(2)-Gluc formation was less affected and other UGT contribute to N(2)-glucuronidation of HONH-AαC. UGT were reported to catalyze the formation of isomeric Gluc conjugates at the N(2) and N3 atoms of 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine (HONH-PhIP), the genotoxic metabolite of PhIP. However, we found that the N3-Gluc of HONH-PhIP also covalently bound to DNA at higher levels than HONH-PhIP. The product ion spectra of this Gluc conjugate acquired by ion trap mass spectrometry revealed that the Gluc moiety was linked to the oxygen atom of HONH-PhIP and not the N3 imidazole atom of the oxime tautomer of HONH-PhIP as was originally proposed. UGT1A9, an abundant UGT isoform expressed in human liver and kidney, preferentially forms the O-linked Gluc conjugates of HONH-AαC and HONH-PhIP as opposed to their detoxicated N(2)-Gluc isomers. The regioselective O-glucuronidation of HONH-AαC and HONH-PhIP, by UGT1A9, is a mechanism of bioactivation of these ubiquitous HAAs.
Figures
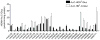




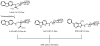
Similar articles
-
Metabolism of the Tobacco Carcinogen 2-Amino-9H-pyrido[2,3-b]indole (AαC) in Primary Human Hepatocytes.Chem Res Toxicol. 2017 Feb 20;30(2):657-668. doi: 10.1021/acs.chemrestox.6b00394. Epub 2016 Dec 15. Chem Res Toxicol. 2017. PMID: 27976871 Free PMC article.
-
UDP-glucuronosyltransferase-mediated metabolic activation of the tobacco carcinogen 2-amino-9H-pyrido[2,3-b]indole.J Biol Chem. 2012 Apr 27;287(18):14960-72. doi: 10.1074/jbc.M111.320093. Epub 2012 Mar 5. J Biol Chem. 2012. PMID: 22393056 Free PMC article.
-
Methemoglobin Formation and Characterization of Hemoglobin Adducts of Carcinogenic Aromatic Amines and Heterocyclic Aromatic Amines.Chem Res Toxicol. 2016 Mar 21;29(3):255-69. doi: 10.1021/acs.chemrestox.5b00418. Epub 2016 Feb 22. Chem Res Toxicol. 2016. PMID: 26824300 Free PMC article.
-
The role of genetic polymorphisms in metabolism of carcinogenic heterocyclic aromatic amines.Curr Drug Metab. 2004 Apr;5(2):169-80. doi: 10.2174/1389200043489036. Curr Drug Metab. 2004. PMID: 15078194 Review.
-
Metabolism of the food carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the rat and other rodents.Princess Takamatsu Symp. 1995;23:113-22. Princess Takamatsu Symp. 1995. PMID: 8844802 Review.
Cited by
-
Prevalence and patterns of mutations in RAS/RAF/MEK/ERK/MAPK signaling pathway in colorectal cancer in North Africa.BMC Cancer. 2022 Nov 7;22(1):1142. doi: 10.1186/s12885-022-10235-w. BMC Cancer. 2022. PMID: 36344948 Free PMC article. Review.
-
Biomonitoring an albumin adduct of the cooked meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in humans.Carcinogenesis. 2018 Dec 31;39(12):1455-1462. doi: 10.1093/carcin/bgy125. Carcinogenesis. 2018. PMID: 30247550 Free PMC article.
-
The development and prevalidation of an in vitro mutagenicity assay based on MutaMouse primary hepatocytes, Part II: Assay performance for the identification of mutagenic chemicals.Environ Mol Mutagen. 2019 May;60(4):348-360. doi: 10.1002/em.22277. Epub 2019 Feb 25. Environ Mol Mutagen. 2019. PMID: 30714215 Free PMC article.
-
The development and prevalidation of an in vitro mutagenicity assay based on MutaMouse primary hepatocytes, Part I: Isolation, structural, genetic, and biochemical characterization.Environ Mol Mutagen. 2019 May;60(4):331-347. doi: 10.1002/em.22253. Epub 2018 Dec 27. Environ Mol Mutagen. 2019. PMID: 30592088 Free PMC article.
-
Metabolism of the Tobacco Carcinogen 2-Amino-9H-pyrido[2,3-b]indole (AαC) in Primary Human Hepatocytes.Chem Res Toxicol. 2017 Feb 20;30(2):657-668. doi: 10.1021/acs.chemrestox.6b00394. Epub 2016 Dec 15. Chem Res Toxicol. 2017. PMID: 27976871 Free PMC article.
References
-
- Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. - PubMed
-
- Yoshida D, Matsumoto T, Yoshimura R, Matsuzaki T. Mutagenicity of amino-alpha-carbolines in pyrolysis products of soybean globulin. Biochem Biophys Res Commun. 1978;83:915–920. - PubMed
-
- Matsumoto T, Yoshida D, Tomita H. Determination of mutagens, amino-alpha-carbolines in grilled foods and cigarette smoke condensate. Cancer Lett. 1981;12:105–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

