The Swiss Multiple Sclerosis Cohort-Study (SMSC): A Prospective Swiss Wide Investigation of Key Phases in Disease Evolution and New Treatment Options
- PMID: 27032105
- PMCID: PMC4816556
- DOI: 10.1371/journal.pone.0152347
The Swiss Multiple Sclerosis Cohort-Study (SMSC): A Prospective Swiss Wide Investigation of Key Phases in Disease Evolution and New Treatment Options
Abstract
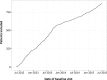
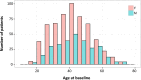
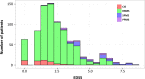
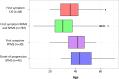
The mechanisms leading to disability and the long-term efficacy and safety of disease modifying drugs (DMDs) in multiple sclerosis (MS) are unclear. We aimed at building a prospective cohort of MS patients with standardized collection of demographic, clinical, MRI data and body fluids that can be used to develop prognostic indicators and biomarkers of disease evolution and therapeutic response. The Swiss MS Cohort (SMSC) is a prospective observational study performed across seven Swiss MS centers including patients with MS, clinically isolated syndrome (CIS), radiologically isolated syndrome or neuromyelitis optica. Neurological and radiological assessments and biological samples are collected every 6-12 months. We recruited 872 patients (clinically isolated syndrome [CIS] 5.5%, relapsing-remitting MS [RRMS] 85.8%, primary progressive MS [PPMS] 3.5%, secondary progressive MS [SPMS] 5.2%) between June 2012 and July 2015. We performed 2,286 visits (median follow-up 398 days) and collected 2,274 serum, plasma and blood samples, 152 cerebrospinal fluid samples and 1,276 brain MRI scans. 158 relapses occurred and expanded disability status scale (EDSS) scores increased in PPMS, SPMS and RRMS patients experiencing relapses. Most RRMS patients were treated with fingolimod (33.4%), natalizumab (24.5%) or injectable DMDs (13.6%). The SMSC will provide relevant information regarding DMDs efficacy and safety and will serve as a comprehensive infrastructure available for nested research projects.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

